Indexed In
- Open J Gate
- JournalTOCs
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2019) Volume 7, Issue 2
Pharmacokinetic and Pharmacodynamic Interaction of Andrographolide with Meloxicam in Wistar Rats
Mythili Srinivasan1, Sathiyanarayanan Lohidasan2, Arulmozhi Sinnathambi3 and Kakasaheb Mahadik2*2Department of Pharmaceutical Chemistry, Poona College of Pharmacy, Bharati Vidyapeeth Deemed to be University (BVDU), Pune 411038, India
3Department of Pharmacology, Poona College of Pharmacy, Bharati Vidyapeeth Deemed to be University (BVDU), Pune 411038, India
Received: 23-Oct-2019 Published: 11-Dec-2019
Abstract
Aim of the study: The primary aim of this study was to evaluate the possible pharmacokinetic and pharmacodynamics (anti-inflammatory) herb-drug interaction of Andrographolide (AN) with meloxicam (Melx) in Wistar rats. Materials and methods: A sensitive and validated RP-HPLC method was developed for the simultaneous estimation of AN and Melx in rat plasma. The oral administration of AN (60mg/kg), Melx 1.55mg/kg) and co-admin group in male-Wistar rats were given. The plasma drug concentration was evaluated using the RP-HPLC method and the pharmacokinetic parameters such as Cmax, Tmax, MRT, T½, CL, Vd, and AUC were calculated. Pharmacodynamic parameters such as Change in paw volume, mechanical hyperalgesia, and mechanical nociceptive threshold were evaluated for predicting the herb-drug interaction.
Results: In the pharmacokinetic study there was a considerable increase in the Cmax, Tmax, MRT, and T½ of the co-admin (AN+Melx) group when compared with the individually administered groups (AN, Melx). On the contrary, there was a significant reduction in the clearance, whereas Vd remains unaffected. In pharmacodynamics studies, co-admin (AN+Melx) groups were showing a significant increase in the anti-inflammatory activity against the disease control group when compared with the individually drug administered groups (AN, Melx).
Conclusion: The results of this study revealed that the co-admin group exhibited herb-drug interactions by giving a twofold reduction of inflammation when compared with the individually drug administered groups. Medical practitioners and patients should get awareness about the potential HDI's of AN with Melx during the concomitant administration of both drugs to avoid undesirable side effects and drug-related toxicities.
Keywords
Andrographolide; Herb-drug interaction; Anti-inflammatory; Pharmacokinetics; Pharmacodynamics; RP-HPLC; Andrographolide (Pub chem CID-5318517); Meloxicam (Compound CID-54677470)
Abbreviations
AN: Andrographolide; Melx: Meloxicam; RP-HPLC: Reverse-Phase High-Performance Liquid Chromatography; HPLC: High-Performance Liquid Chromatography; Cmax: Maximum Plasma Concentration; Tmax: Time Needed to Reach Concentration Maximum; T½: Elimination Half-Life; AUC0-T: Area Under Plasma Concentration–From Zero to End Time; AUC 0-∞: Area under Plasma Concentration-Time Curve From Zero to Infinity; CL: Clearance from the Plasma; ANOVA: Analysis of Variance; CYP: Cytochrome P450; MRT 0-T: Mean Residence Time From Zero Last Time Point; MRT 0-∞: Mean Residence Time Till Infinity; NSAID: Non-Steroidal Anti-Inflammatory Drug; SEM: Standard Error of the Mean; SD: Standard Deviation; R: Correlation Coefficient; Vd: Apparent Volume of Distribution; ADME: Adsorption, Distribution, Metabolism, And Excretion; COX2: Cyclooxygenase 2, Tnfα: Tumour Necrotic Factor Α; IL 2: Intra Leukin 2; COPD: Chronic Obstructive Pulmonary Disease; HDI: Herb Drug Interaction; AP: Andrographis paniculata (Nees)
Introduction
Andrographis paniculata (Nees) (AP) belonging to the family Acanthaceae is an annual herb mainly distributed in the tropical Asian countries like India, Indonesia, Sri Lanka, Pakistan, and Malaysia. It is also cultivated extensively in China, Thailand, Mauritius, East and West Indies. It is found in a variety of habitats like hill slopes, wetlands, farms, riversides, and roadside. The plant is synonymous with the names The Creat, King of Bitters (English); Kiryat (Hindi); Kalmegha (Sanskrit), Quasabhuva (Arab); and Nilavembu (Tamil). The plant is extensively used in traditional medical systems such as Ayurveda, Siddha, and Unani and Chinese herbal system of medicine. The plant is long been used orally to prevent and treat upper respiratory tract infections, inflammation, common cold, pharyngotonsillitis and sinusitis [1]. In the current scenario, it is used as an antidiabetic, antihypertensive, anticancer, immunomodulatory and hepatoprotective agent. After conducting a wide array of studies the modern usage of this plant is extended for anti-dengue, anti-cancer, and anti-HIV activities [2]. AP contains diterpenoid lactones known as terpenoid compounds that have been isolated from the aerial parts and roots of this plant. The predominant diterpenoid compound in terms of abundance and quantity is andrographolide. Andrographolide, a crystalline powder, is extremely bitter in taste. The main phytoconstituents of this plant include Andrographolide(AN), neo Andrographolide, 14, deoxy 11, 12 di dehydro andrographolide, Bisandrographolides A, B, C and D(diterpene dimer) and a flavonoid called 5,7,2',3'-tetramethoxyflavanone and 5-hydroxy-7,2',3'-trimethoxy flavone.
AP is the long-used medicinal herb for treating inflammation and inflammation-related diseases like diabetes, COPD, Rheumatoid arthritis, inflammatory bowel diseases, and cancer. The most possible mechanism of the anti-inflammatory activity is achieved through COX-2 inhibition, suppression of nitric-oxide synthase and TNF alpha and IL-12, IL-8 regression.
It is a common practice to use herbs alongside synthetic NSAIDs such as Meloxicam and Mefenamic acid in the treatment of inflammatory diseases. The mechanism involved with meloxicam is the inhibition of enzyme cyclooxygenase (COX) that is responsible for converting arachidonic acid into prostaglandin H2. This is the primary step for the synthesis of mediators of inflammatory i.e., prostaglandins. In the case of meloxicam, even at a lower therapeutic dose, this will selectively inhibit cyclooxygenase-2 (COX-2) over cyclooxygenase-1 (COX-1) without affecting platelet aggregation [3]. AP is available as alternative medicine in Indian and Chinese local markets. Most of the time, herbal medicines are taken as complementary alternative medicine (CAM) with or without the knowledge of medical practitioners. This concomitant administration may sometimes lead to potential herbdrug interactions causing synergistic or additive or antagonistic effects. Herb-drug interactions (HDIs) are mediated by the Cytochrome p450 group of enzymes, Para-glycoprotein and both. Cytochrome p450 is the group of isoenzymes responsible for the biotransformation of most of the synthetic drugs. Para-glycoprotein is the receptor mediating protein responsible for the influx and efflux of drugs [4,5]. HDI’s occur when the enzymes responsible for biotransformation are common for both herb and synthetic drugs. The Cyp enzymes responsible for the metabolism of AN is Cyp3A4 and Cyp2C9. The same cytochromes are involved with the biotransformation of the oxicam group of compounds. This common Cyp enzymes involved in the biotransformation of AN and Melx is giving speculations about the possible interactions between these two drugs. Many studies have been carried out to predict the pharmacokinetic HDI's. Several studies have been published on HDI's of AN with other conventional drugs. Unfortunately, no single study has been carried out for the HDI prediction of Meloxicam with Andrographolide [6,7]. Since AN is the most active ingredient of AP and also potent anti-inflammatory drug it was decided to evaluate the effect of AN and Melx on the pharmacokinetics of each other. To predict the pharmacokinetic interaction of each drug a bioanalytical chromatography method for the simultaneous estimation of AN and Melx in rat plasma will be more suitable. Unfortunately, no such methods are available yet for the simultaneous estimation of AN and Melx. Hence the objective of the present study was to investigate the pharmacokinetic and pharmacodynamic interactions of AN and Melx when co-administered in Wistar rats.
Materials and Methods
Chemicals and reagents
Andrographolide was purchased from Mayser herbals, Faridabad, Haryana. Meloxicam BP (CAS number-71125-38-7) was obtained as a generous gift from Ramdev chemical Pvt Ltd, Raipur, Boisar, Palghar district. HPLC grade methanol was purchased from Merck Chemicals, Mumbai, Maharashtra, India. High purity deionized water was obtained from Millipore, Milli-Q (Bedford, MA, USA) water purification system. Perchloric acid 70% was purchased from Merck Chemicals, Mumbai, Maharashtra, India.
Animals
Male Wistar rats weighing 220-250 g were purchased from the National Institute of Biosciences, Dhangawadi, Maharashtra. In a single cage six rats were placed and maintained under controlled room temperature (25 ± 2°C) and humidity (60-70%) with a twelvehour day/night cycle. All animals had provided proper food and water. After acclimatization for one week animals were fasted overnight (12 h) before each experiment. All animal experiments were performed as per the guidelines of CPCSEA after obtaining approval (CPCSEA/PCP/PCH06/2018) from the Institutional Animal Ethics Committee.
Chromatographic analysis
Before finalizing the mobile phase number of trials are performed to optimize the method for simultaneous estimation of AN and Melx by using various solvents of different concentrations which include methanol, water, ACN and buffers like Tris-acetate, Disodium hydrogen phosphate and Potassium phosphate and Ammonium acetate were used. The chromatographic separation was performed using an RP-HPLC method with a Jasco PU-1580 Isocratic liquid chromatography instrument and a UV detector UV-1575. A C18 Hypersil Gold (250 × 10 mm id, 5 μm) was used for separation [8,9]. The mobile phase system was optimized to give a good resolution of AN and Melx from other endogenous substances in the plasma sample.
Preparation of stock solutions and calibration samples
The stock solution was prepared by dissolving an accurate amount of reference standards in DMF and methanol at a concentration of 1 mg/ml for Andrographolide and Meloxicam (Figure 1). A series of working standard solutions were obtained by further diluting the stock solution in methanol. Calibration standards were prepared by spiking the appropriate amounts of the standard solutions into 200 µl of blank plasma to yield final concentrations of 1, 3, and 5 and up to 25 μg/ml. The quality control (QC) samples were similarly prepared at concentrations of 9 and 15 and 23 µg/ml for low, medium and high concentration ranges respectively. All solutions were kept refrigerated and brought to room temperature before use.
Figure 1: Compound structures of A. Andrographolide, B. Meloxicam (Source-Pubchem).
Plasma sample preparation
Plasma samples (250 µl) were added with 1 ml of 7% per chloric acid and methanol (90:10). The prepared samples were centrifuged at 15000 rpm at -4°C for 20 minutes. The supernatant was collected and reconstituted with 500 µl of the mobile phase. The sample was vortexed for 2 to 3 minutes. Filtered and 20 µl aliquot was injected for HPLC analysis. The quality control (QC) samples were prepared similarly on a bulk based on an independent weighing of standard drugs, at three concentrations (9, 15, 23 µg/ml) as a single batch at each concentration.
Method Validation
The developed HPLC method was validated as per the USFDA guidelines for linearity, precision, drug recovery, the limit of detection (LOD), Lower level of quantification (LLOQ) and robustness.
Linearity
The linear regression data for the calibration curve (n=13) is checked for a good linear relationship over the concentration range of 1 µg/ml-25 µg/ml for both AN and Melx. The value of the significance and correlation coefficients confirm the linearity in the selected concentration range.
LOD and LLOQ
The limit of detection and Lower limit of quantification was calculated for AN and Melx based on the equation, LOD=3.3(SD)/S and LLOQ=10(SD)/S according to the guidelines, Where, SD=standard deviation of the response and S=slope of the regression equation.
Precision
The precision of the method was verified by inter-day and intra-day precision. It was evaluated by six replicate analysis of the composite sample. The %RSDcalculated for Inter-day and Intra-day for both AN and Melx, following USFDA guidelines which recommend %RSD should be below 20.
Repeatability
Repeatability of the method was checked by using the standard solution of AN and Melx with a concentration of 9 µg/ml, 15 μg/ml and 23 μg/ml. The percent RSD should be <20% as per guidelines.
Recovery
The drug recovery study of AN and Melx was carried out by standard addition method in which three different concentration of the API was spiked additionally to get a solution of 80%, 100%, and 120%. The percentage recovery was calculated using the recovered drug to that of the added drug.
Robustness
Robustness examines the effect of the operational parameters on the analysis results. Small deliberate changes in some parameters such as wavelength, flow rate, and pH were introduced to check the method reliability. The deviation obtained by deliberate changes in the mentioned parameters must be below 20% RSD as per guidelines.
Stability
Freeze-thaw stability and benchtop stability was performed. Freezethaw stability was done with three concentrations of 9 µg/ml, 15 μg/ml and 23 μg/ml by using 24 hours freeze-thaw cycle. Three consecutive freeze-thaw cycles were performed and significant changes were measured and reported. Benchtop stability was performed by introducing the samples to a regular laboratory working condition.
Pharmacokinetic study
Drug administration: The dosage of AN was calculated as per previous literature according to body weight. Acute toxicity studies were already performed as per OECD guidelines. Hence, the dose is calculated for AN as per previous studies [7,10,11]. In the case of Meloxicam, the dosage was calculated by converting the human dose into animal dosage as per body surface area by either multiplying or dividing with the conversion factor with the given body weight. The conversion factors were given in previous literature [12]. Experimental animals were randomly divided into four groups of 12 animals each and received drug treatment orally as follows. Group 1-Vehicle control, Group 2-AN alone (60 mg/ kg of b.w.) Group 3-Melx alone with a dose of 1.55 mg/kg/b.w. [12] and Group 4-Co-administration of AN with Melx (60 mg/ kg+1.55 mg/kg, b.w.) as a single dose. Doses were selected as per the previous literature [7,10,11]. After drug administration, 12 animals were further subdivided into two groups with six animals each.
Blood sampling: Blood samples (1 ml each) were collected at 15 min, 1, 2.30, 4.45 and 12 hours in first subgroup 2, 4, 7, 24 and 48 hours in the second subgroup. Only one blood sample was collected from the individual animals within 24 hours from the retro-orbital plexus under light ether anesthesia. The samples were transferred to EDTA tubes and centrifuged at 15000 rpm at 4°C for 15min. Plasma was separated from the blood and stored at -80°C until further analysis.
Pharmacodynamic studies
Male Wistar rats weighing 220-250 g were purchased from the National Institute of Biosciences. Six rats were placed in one cage and maintained under controlled room temperature (25±2°C) and humidity (60-70%) with the day/night cycle (12 h/12 h). All animals were allowed to get free access to food and water. After acclimatization for one week animals were fasted overnight (12 h) before each experiment. All experiments were performed as per the guidelines of CPCSEA after obtaining approval (CPCSEA/PCP/ PCH06/2018) from the Institutional Animal Ethics Committee. Animals were divided into five groups of 6 animals each. The first group is a normal control group, the second one is disease control, third group is treated only with AN (60 mg/kg of BW), [11] fourth group is Melx alone treated group (1.55 mg/kg of BW) [12] and the fifth one is Co admin group (AN (60 mg/kg)+Melx (1.55 mg/ kg of BW)).
Induction of inflammation: Hind-paw edema was induced by a sub plantar injection of 0.1 ml Carrageenan (1% w/v) in water. The prepared sample of 0.1 mL of 1 % w/v carrageenan was injected into the sub plantar tissue of the left hind paw of each rat. Drugs were administered orally 30 minutes before disease induction. After disease induction parameters like Change in paw volume, Mechanical hyperalgesia and mechanical nociceptive threshold were evaluated [13,14].
Paw volume: The swelling of the carrageenan injected foot was measured at 3, 4, and 5 hours using Plethysmometer (UGO Basile, Italy). Animals were treated with andrographolide, Meloxicam and both before 30 minutes of the carrageenan injection [13,14]. Measurement was carried out immediately before and after 3hrs following carrageenan injection. Percent inhibition of test drugs was calculated in comparison with the disease control (100%) [11,15].
Mechanical hyperalgesia: Mechanical hyperalgesia of left hind paw was evaluated by Randall and Selitto test using an analgesiometer (UGO Basile, Italy). The cut-off pressure was 450 g. Radiant heat was positioned directly beneath the hind paw of the chamber floor. The latency to paw withdrawal was recorded automatically by a photocell and an electronic timer. To avoid tissue damage, the intensity of the radiant heat was adjusted to achieve baseline latencies of 10-15 sec and a cut-off time of 15 sec. The animals which are unresponsive were discarded after 30 sec (cut-off time). For each animal, two subsequent applications of the heating stimulus were applied, separated by 1-2 min intervals, and the mean of the two measures; Paw withdrawal latencies were recorded before carrageenan administration and after 3,4,5,6 hours. The responses against inflammation were measured concerning disease control [7,15].
Mechanical nociceptive threshold: The nociceptive threshold to mechanical stimulation was determined using Von Frey hairs (ALMEMO, Germany) of the increasing gauge. The animals were allowed to acclimatize for 10 min in the Perspex box and Von Frey hairs (0.6 to 12.6 g) were applied to the plantar surface of the left hind paw. [15] A series of three stimuli were applied to each paw for each hair within a period of 2-3 s. The lowest weight of Von Frey hair to evoke a withdrawal from the three consecutive applications was considered to indicate the threshold. The mean withdrawal of test groups was compared with disease control [7].
Statistical Analysis
For the pharmacokinetic study, the plasma concentrations versus time profiles of individual animals were estimated by a non-compartmental model using Pk solver software (Pharsight Corporation, Mountain View, CA, USA). All data were expressed as mean ± SD. Differences between groups were evaluated by twoway ANOVA (Bonferroni test) using GraphPad Prism software (San Diego, California, USA). The differences were considered to be significant at *P<0.05, **P<0.01, ***P<0.001.
Results
Method development using HPLC
Various trials were performed using different solvents of varying concentrations includes methanol, ACN, water and buffers like phosphate buffer, Ammonium acetate and Tris acetate buffers before finalizing the method. The chromatographic separation was performed using an RP-HPLC method with a Jasco PU-1580 Isocratic liquid chromatography instrument and a UV detector UV-1575. A C18 Hypersil Gold (250 × 10 mm id, 5 μm) was used for separation [8,9]. The mobile phase system was optimized to give a good resolution of AN and Melx from other endogenous substances in the plasma sample. Sodium dihydrogen phosphate buffer pH 7.5 and methanol of 50:50 v/v was used as a mobile phase [16]. The pH of the mobile phase was adjusted with 0.1 M Sodium hydroxide. The run time was 20 minutes at a flow rate of 1 ml/min and a detection wavelength of 242 nm. Each calibration curve was analysed individually by using the least square weight linear regression equations. Validation of the developed method was done according to the US Food and Drug Administration (FDA) guidelines. (US Food and Drug Administration, Center for Drug Evaluation and Research [17]. The developed HPLC method was found to separate AN and Melx successfully with the retention time of 7.03 and 9.9 min respectively (Figure 2).
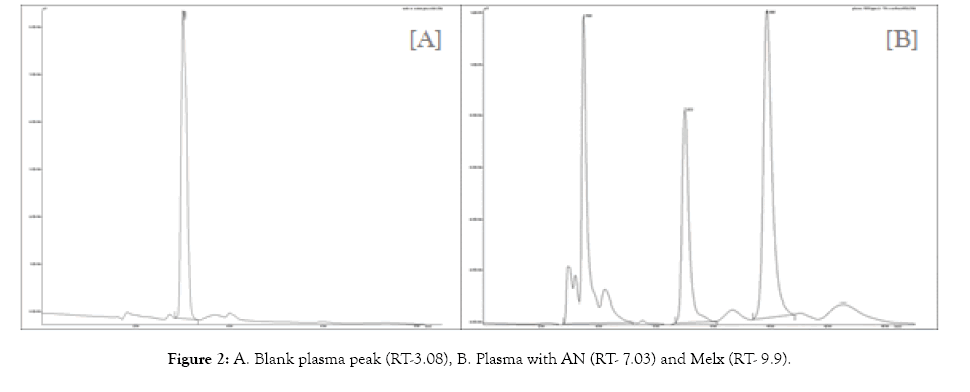
Figure 2: A. Blank plasma peak (RT-3.08), B. Plasma with AN (RT- 7.03) and Melx (RT- 9.9).
Method validation
Linearity: Linear regression data for the calibration curve (n=13) showed a good linear relationship over the concentration range of 1-25 µg/ml for AN and Melx (correlation coefficient r=0.993 for AN and r=0.996 for Melx), the data were subjected to regression analysis. The value of the significance and correlation co-efficient confirm the linearity in the selected concentration range which is depicted in (Figure 3).
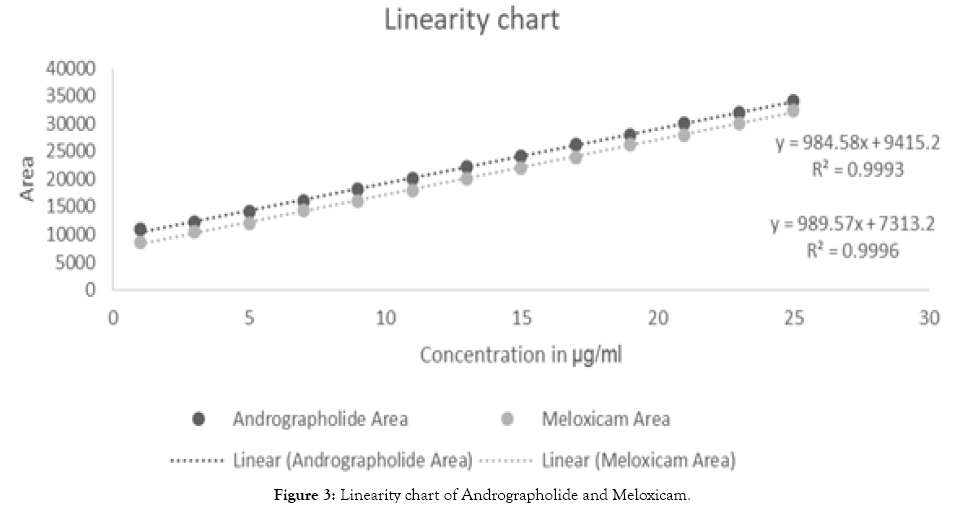
Figure 3: Linearity chart of Andrographolide and Meloxicam.
LOD and LLOQ: The limit of detection and Lower level of quantification was calculated for AN and Melx based on the equation, LOD=3.3 (SD)/S and LLOQ=10 (SD)/S according to the guidelines, Where, SD=standard deviation of the response and S=slope of the regression equation. The level of detection and level of quantification is calculated using 1 µg/ml concentration of drug in plasma and the LOD was found to be 22 ng/ml for AN and 23 ng/ml for Melx. The lower limit of quantification was found to be 66 ng/ml for AN and 70 ng/ml for Melx.
Precision: The precision of the method was evaluated by inter-day and intra-day precision with six replicate analysis of the standard solution of AN and Melx for three different concentrations of 9, 15 and 23 µg/ml. The developed method was found to be precise as the RSD values for intra-day and inter-day precision have complied with the given limits of USFDA guidelines. The percentage RSD calculated was found to be below 4 for Inter-day and below 3.5 for Intra-day for both AN and Melx.
Repeatability: Repeatability of the method was checked by analysing the standard solution of AN and Melx with a concentration range of 9 µg/ml, 15 µg/ml and 23 µg/ml with six replicates (n=6) and the percent RSD For AN and Melx was found to be 1.5, 0.13, 0.3 and 0.71, 0.08, 0.39 respectively. The percent RSD should be <15% as per guidelines.
Recovery: The recovery study of AN and Melx was carried out by standard addition method in which three different concentration of the API was spiked additionally to get a solution of three different concentration of 80%, 100% and 120% of AN and Melx and the total amount of the drug recovered was determined. The results were found to be 96 to 98.9% for both drugs and it is within the range of 85 to 115% complying the guidelines.
Robustness: Robustness studies were done by making small, deliberate changes in an optimized condition like change in flow rate, change in detection wavelength, and change in pH, etc. The percentage RSD was found to be below 2.3% and 3.4% for AN and Melx respectively and which is <20% complying the acceptable range as per guidelines.
Stability: Freeze-thaw stability and benchtop stability were performed. Freeze-thaw stability was performed for three concentrations 9, 15 and 23 µg/ml and with three replicates (n=3). The percentage RSD was found to be 0.01-0.04 for AN and 0.01-1.21 for Melx. Benchtop stability was performed for three concentrations 9, 15 and 23 µg/ml and with three replicates (n=3). The percentage RSD was found to be 0.008-1.54 for AN and 0.007- 1.46 for Melx. Both values are ˂15% and are in the acceptable range as per guidelines.
In vivo pharmacokinetic study
After a successful HPLC evaluation, the pharmacokinetic parameters were analysed using pk solver software (pharsight, Italy). There was a significant difference between the Co-admin group when comparing with the individually administered groups which are shown in (Tables 1 and 2). When comparing AN with AN co-admin group, there is almost 61.1% difference in T½ (P<0.01), 62% difference in MRT (P<0.01) and 23.8% difference in Vd. Moreover, all the above-stated parameters were increasing when comparing with the AN alone group. On the contrary, the clearance level is considerably decreasing by 43.63% (P<0.05) when comparing with the AN alone group shown in (Figure 4). On the other hand, in Melx alone and Melx co-admin group, there was almost 6.54% (P<0.05) difference in T½, 14.83% difference in Tmax, 7.3% difference in Cmax, 6.9% difference in Vd and 0.3% difference in Clearance. Moreover, all the above-stated parameters were increasing when comparing with Melx alone group. Whereas the AUC 0-t and AUC0-∞ was decreasing considerably with the difference of 4.12% and 0.5% respectively when comparing with Melx alone group shown in (Figure 5).
| Parameters | Unit | AN alone | AN co-admin |
|---|---|---|---|
| t1/2 | h | 4.47 ± 0.43 | 18.54 ± 0.06** |
| Tmax | h | 2.3 ± 0.01 | 4 ± 0.016 |
| Cmax | mg/ml | 0.162 ± 0.04 | 0.19 ± 0.00 |
| AUC 0-t | mg/ml*h | 1.15 ± 0.04 | 2.4 ± 0.069 |
| AUC 0-inf_obs | mg/ml*h | 1.16 ± 0.08 | 2.95 ± 0.059 |
| MRT 0-inf_obs | h | 5.65 ± 0.02 | 24.12 ± 0.017* |
| Vz/F_obs | (mg/kg)/(mg/ml) | 334.12 ± 0.084 | 543.09 ± 0.07* |
| Cl/F_obs | (mg/kg)/(mg/ml)/h | 51.69 ± 0.09 | 20.29 ± 0.043* |
Differences between groups were analysed by two way ANOVA followed by Bonferroni test using Graphpad prism software (San Diego, California, USA). Data are expressed Mean ± SD; n=12 rats per group to be significant at *P<0.05, **P<0.01, ***P<0.001 Compared to AN alone group.
Table 1: Results of pharmacokinetic parameters of AN vs. AN co-administered group.
| Parameters | Unit | Melx alone | Melx co-admin |
|---|---|---|---|
| t1/2 | h | 23.96 ± 0.092 | 27.25 ± 0.041* |
| Tmax | h | 4.45 ± 0.026 | 6 ± 0.03 |
| Cmax | mg/ml | 0.32 ± 0.033 | 0.37 ± 0.00 |
| AUC 0-t | mg/ml*h | 7.38 ± 0.029 | 5.72 ± 0.05 |
| AUC 0-inf_obs | mg/ml*h | 9.80 ± 0.035 | 9.71 ± 0.032 |
| MRT 0-inf_obs | h | 33.89 ± 0.07 | 39.26 ± 0.02* |
| Vz/F_obs | (mg/kg)/(mg/ml) | 5.46 ± 0.073 | 6.27 ± 0.043 |
| Cl/F_obs | (mg/kg)/(mg/ml)/h | 0.1581 ± 0.00 | 0.159 ± 0.00 |
Differences between groups were analysed by two way ANOVA followed by Bonferroni test using Graphpad prism software (San Diego, California, USA). Data are expressed Mean ± SD; n=12 rats per group to be significant at *P<0.05, **P<0.01, ***P<0.001 Compared to Melx alone group.
Table 2: Results of pharmacokinetic parameters of Melx vs. Melx co-administered group.
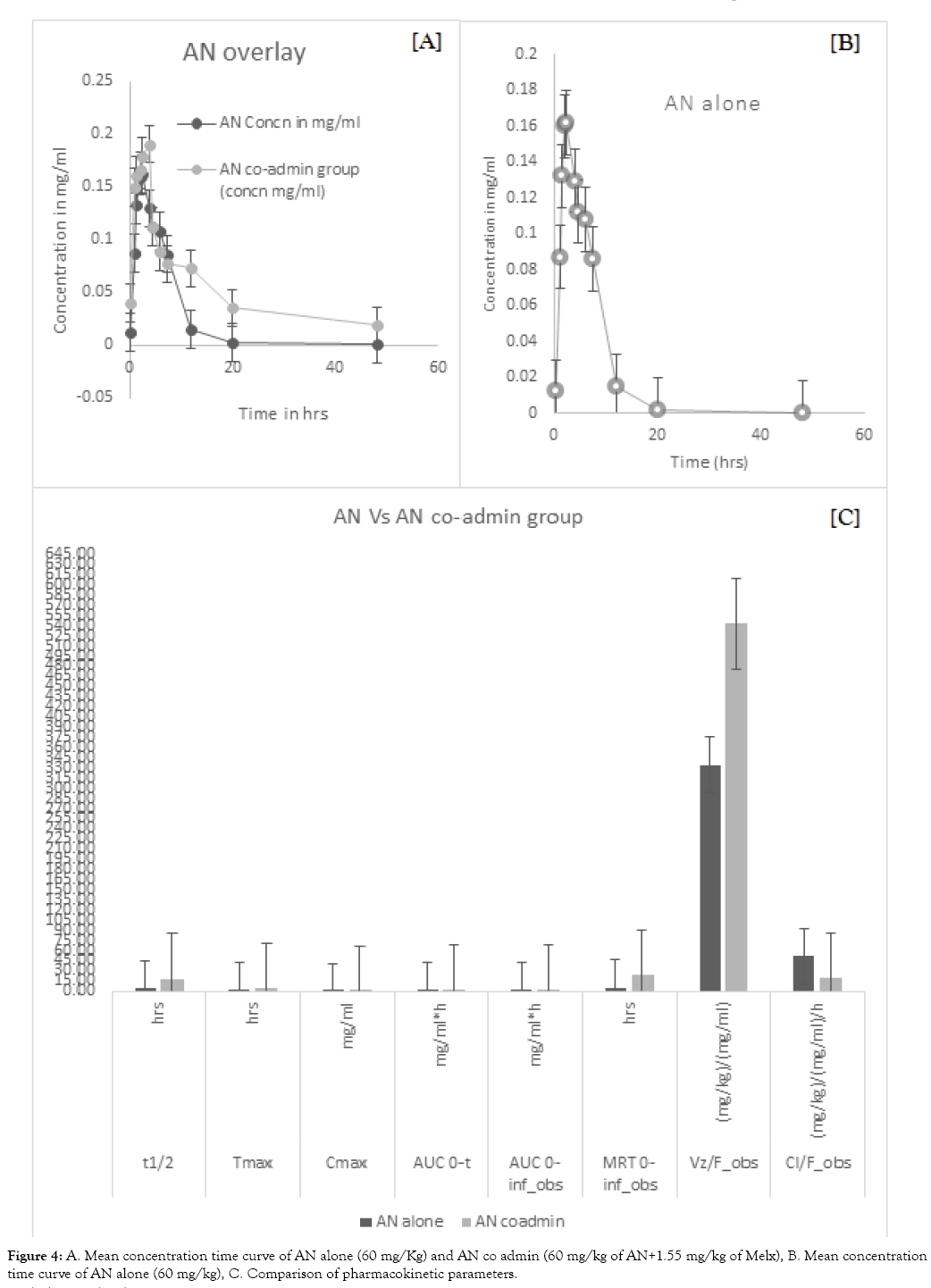
Figure 4: A. Mean concentration time curve of AN alone (60 mg/Kg) and AN co admin (60 mg/kg of AN+1.55 mg/kg of Melx), B. Mean concentra tion time curve of AN alone (60 mg/kg), C. Comparison of pharmacokinetic parameters.
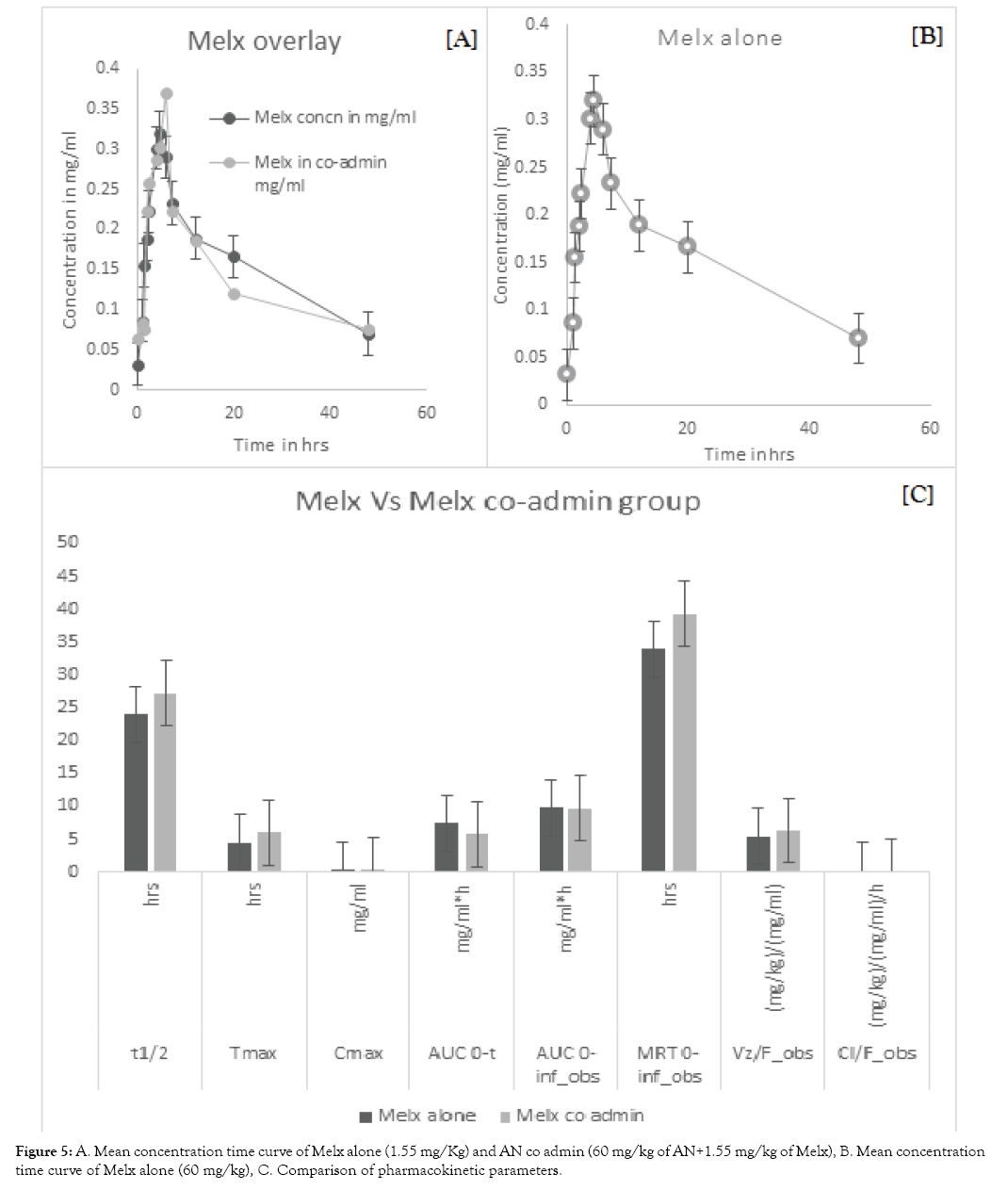
Figure 5: A. Mean concentration time curve of Melx alone (1.55 mg/Kg) and AN co admin (60 mg/kg of AN+1.55 mg/kg of Melx), B. Mean concentration time curve of Melx alone (60 mg/kg), C. Comparison of pharmacokinetic parameters.
On the whole, there were significant changes in T½ and MRT of the co-admin group indicating that the drug was remaining more time in the body. In addition to this, clearance was considerably decreasing. Though there was no statistical significance, the parameters like Cmax, Tmax, AUC 0-t and AUC0-∞ were increasing considerably in the AN co-admin group. In the case of Melx co-admin other than AUC 0-t and AUC0-∞, the remaining parameters were increasing.
Pharmacodynamic studies (Anti-inflammatory)
Change in paw volume: In paw volume studies, the AN alone group was showing significant inhibition at 4 and 5 hrs (P<0.01, P<0.001) respectively. Melx alone was showing greater inhibitory response when compared with the AN alone group (P<0.001). On the other hand, the individually administered groups were showing a relatively lesser effect than that of the co-admin group which is given in (Table 3). The percentage inhibition of the co-admin group is higher in all-time points with significance (P<0.001) when compared with the disease control group. The changes in the paw volume graph are depicted in (Figure 6).
| Time in hrs | Disease control | Normal control | AN alone | Melx alone | Co-admin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | Mean in ml | SEM | Mean in ml | SEM | Mean in ml | SEM | % Inhibition | Mean in ml | SEM | % Inhibition | Mean in ml | SEM | % Inhibition |
| 1 | 6.93± 0.03 | 0.09 | 2.033 ± 0.03 | 0.049 | 6.9 ± 0.08 | 0.08 | 5.50% | 6.65 ± 0.09 | 0.1 | 9% | 6.21 ± 0.06 | 0.13 | 11%*** |
| 2 | 7.1± 0.04 | 0.08 | 2.2 ± 0.05 | 0.044 | 6.5 ± 0.03 | 0.07 | 13%** | 5.88 ± 0.03 | 0.08 | 17.4%*** | 5.0 ± 0.045 | 0.25 | 35%*** |
| 3 | 7.28 ± 0.03 | 0.04 | 2.03 ± 0.03 | 0.071 | 5.8 ± 0.01 | 0.16 | 26%*** | 5.01 ± 0.06 | 0.21 | 37%*** | 2.8 ± 0.03 | 0.08 | 71%*** |
Differences between groups were analysed by two way ANOVA followed by Bonferroni test using Graphpad prism software (San Diego, California, USA). Data are expressed Mean ± SEM; n=6 rats per group. Data gives significance at *P<0.05, **P<0.01, ***P<0.001 compared to disease control.
Table 3: Results of changes in paw volume.
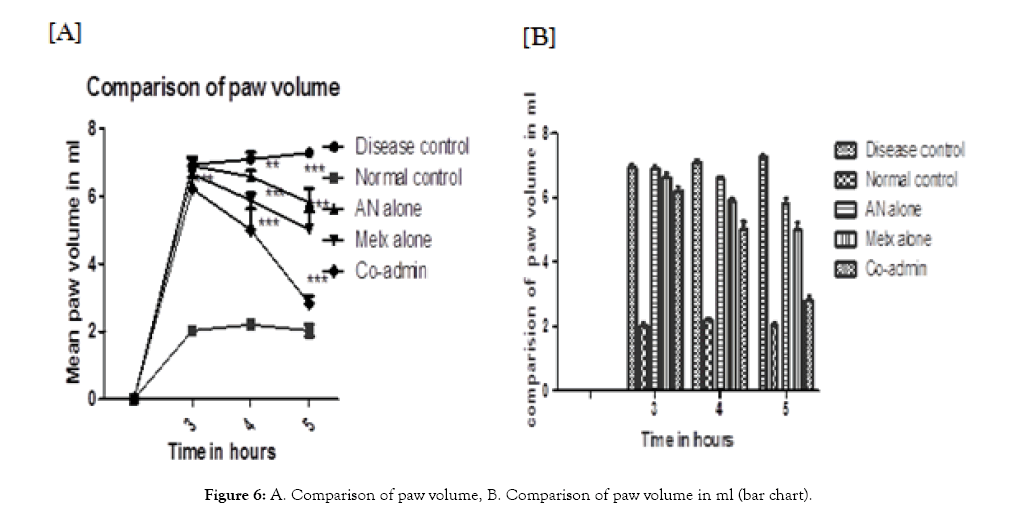
Figure 6: A. Comparison of paw volume, B. Comparison of paw volume in ml (bar chart).
Effect on mechanical hyperalgesia: The paw withdrawal effect was considered in the AN alone group and it was showing significance only in 6 hours (P<0.001) when compared with disease control. In the case of the Melx group the increase in pain, the threshold is comparatively greater than the AN alone group and it was showing significance at 4, 5, and 6 hrs. (P<0.001). Further, the co-admin group was showing a relatively higher response to that of the individually administered groups. It was showing greater response with significance in all time points. All significant changes are shown in (Figure 7).
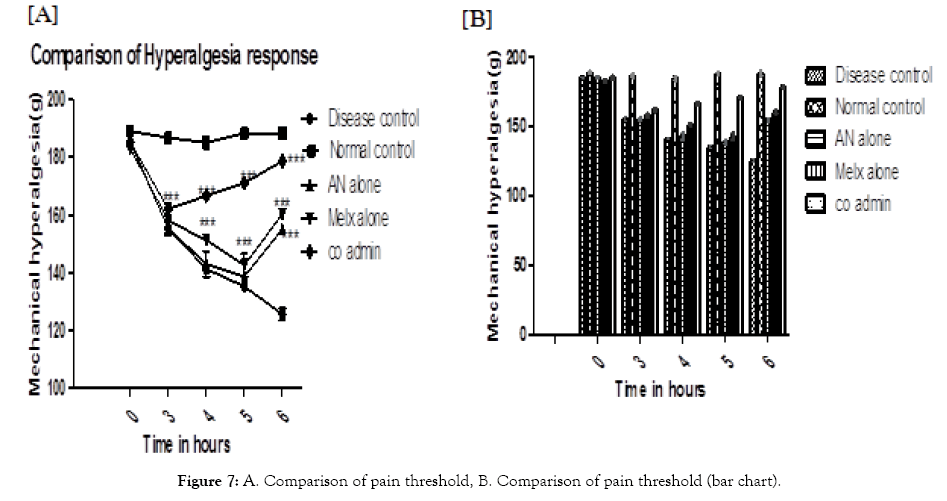
Figure 7: A. Comparison of pain threshold, B. Comparison of pain threshold (bar chart).
Mechanical nociceptive threshold: After carrageenan induction, there was a considerable reduction in the mechanical withdrawal of all disease-induced animals. After 3 hrs. the AN alone group was showing good improvement after 3 hours (P<0.01) with significance and after 5 hours it was showing better significance (P<0.05). Whereas, the Melx group was showing comparatively better improvement in the mechanical withdrawal threshold at 3 and 6 hours (P<0.05), a greater response in 5 hours (P<0.001). In addition to this, the co-admin group was observed with a relatively higher response when compared with individual groups. It was showing significant improvement at 5 and 6 hours (P<0.001). The results were depicted in (Figure 8).
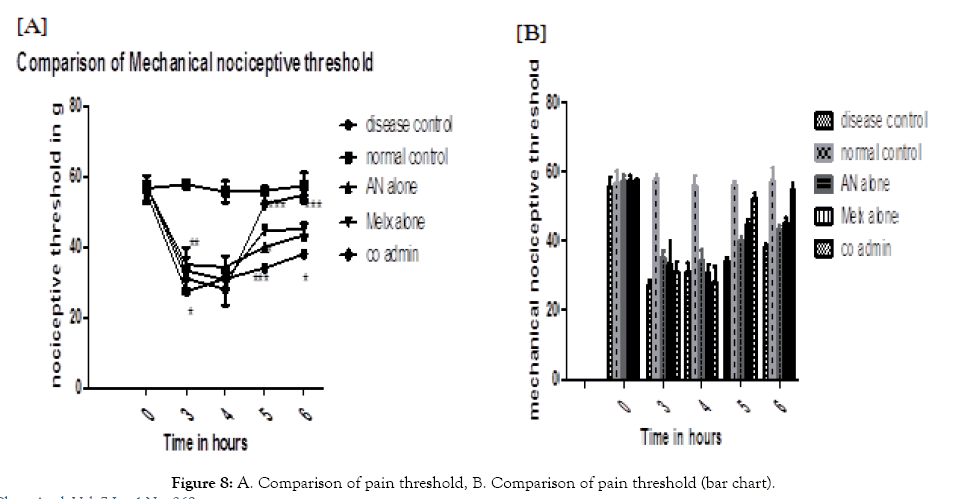
Figure 8: A. Comparison of pain threshold, B. Comparison of pain threshold (bar chart).
Discussion
AN is the predominant chemical constituent of Andrographis paniculata (Nees). It has tremendous pharmacological activities, among which anti-inflammatory activity is well proven one [18]. This herb is useful in treating inflammation and inflammationrelated diseases for decades. Most of the alternative medical systems (Unani, Siddha, and Ayurveda) are using this medicinal herb as a total extract individually or in various polyherbal formulations. [1,2]. In the case of the chronic inflammatory condition, it has become a regular practice to consume complementary alternative medicine, to avoid side effects. These complimentary (CAM) medicines on concomitant usage with synthetic NSAIDS will cause HDI's. [4,19] HDI’s are of two types, pharmacokinetic and pharmacodynamic. Pharmacokinetic HDI's are relating to ADME of the drug along with protein binding and it is regularised by the Cytochrome group of enzymes and para-glycoprotein. Pharmacodynamic HDI's are related to receptor binding and drug displacement in the organ, enzymes, and tissues. The drug interactions related to pharmacokinetic HDI’s are relatively higher and significant. These types of HDI's are very difficult to control and lead to synergistic, additive effects and antagonistic effects (undesirable and toxic effects) [19,20].
Cytochromes are the group of isoenzymes responsible for the biotransformation of 90% of the synthetic drugs. Among these, Cyp3A4 is the predominant one covering 75% of drugs. In the case of AN, the cytochromes responsible for its metabolism is Cyp3A4 and Cyp2C9 [21,22]. The same Cyp’s are involved in the biotransformation of the Oxicam group of drugs [6]. The common Cyp’s are posing HDI related speculations, but in contrary CYP3A4 is absent in rats [23]. However In rats, the Cyp3A11 is matching with human Cyp3A4 with similar amino acid homology and in both cases, the enzyme is expressed in intestine and involved in the biotransformation of CYP3A4 related drugs [23]. Therefore, the most possible interaction is through Cyp3A11 and the most possible mechanism is through non-competitive enzyme inhibition [5]. AN is the substrate drug of Para-glycoprotein and P-gp is the efflux pump controlling the drug permeability through the membrane. After absorption, more amount of AN gets ejected out leading to poor bioavailability giving way to concomitantly administered drugs by improving their bioavailability [10]. In a study AN is improving the Cmax of warfarin in rats [6]. In another study, AN improves the bioavailability of glyburide on concomitant administration in rats [24]. In the present study, AN is increasing the Cmax of Melx however with no statistical significance. In addition to this, AN is increasing the MRT and T½ of Meloxicam with significance (P<0.05) and decreasing the clearance level which shows the longer retainment of Melx in the systemic circulation.
In pharmacodynamic studies, the Hind paw volume parameter analysed by plethysmometer is showing considerable statistical significance with AN alone and Melx alone groups (P<0.001) at 4 and 5 hours, whereas a co-admin group is showing a two-fold reduction in paw volume when comparing with AN alone and Melx alone groups. Moreover, the co-admin group is showing high significance at 3, 4 and 5 hours. In addition to this, Hyperalgesia studies are showing significance only at 6 hours, on the other hand, the Melx group is exhibiting significance at 4, 5, 6 hours (p<0.001). Most predominantly the co-admin group is showing comparatively higher significance than the individually administered groups. This group is giving significance at almost all time points (P<0.001) with double the response when compared with individual groups. Furthermore, in nociceptive threshold studies, when comparing with AN alone and Melx alone groups, the co-admin group is exhibiting a relatively high proportion of response at time points 5 and 6 hours (P<0.001).
From all three behavioral parameters, it is visible that the co-admin group is showing a relatively high degree of effect when compared with individual groups (AN alone and Melx alone). Besides, AN is increasing the T½ and MRT of Meloxicam to a considerable extent with significance (p<0.05). AN is also increasing Cmax and Tmax with no significance (P>0.05). The results of the study can be looked in a way that the improved duration of drugs in systemic circulation leads to an increase in activity however such combination in prolonged treatment should not result in any toxic effects. Hence dose reduction may be considered to get benefits of co-administration. From all the above-stated reasons, this study is showing a good correlation between the pharmacokinetics and pharmacodynamics. Further, there is an improved activity in the co-admin group is giving a clear vision of HDI. On the whole, a detailed HDI related study is needed in humans for ascertaining and correlating the possibilities of HDI.
Conclusion
To conclude, the results obtained from both pharmacokinetic and pharmacodynamic studies are well correlating and indicate the possible interaction of AN with Melx. In the case of Pharmacokinetics, there is an increase in the Tmax, Cmax, T½ and MRT of Melx in the AN co-administered group when compared with the individually administered group. Moreover, the kinetic results are reflected in pharmacodynamic studies, in which there was a two-fold increase in the anti-inflammatory activity of the coadmin group observed when compared with the individual drug administered group. In both cases, the results were significant and giving a clear vision about the possible HDI. Furthermore, an in-depth study is needed in humans to prove and correlate the possible HDI's. This study provides awareness and HDI related knowledge of both drugs AN and Melx when co-administered. The patients and medical practitioners should be aware of this on its rational use in inflammation to prevent undesirable effects.
Conflict of Interest
This research does not have any conflict of interest with anyone or any institute.
REFERENCES
- Chandrasekaran CV, Gupta A, Agarwal A. Effect of an extract of Andrographis paniculata leaves on inflammatory and allergic mediators in vitro. J Ethnopharmacol. 2010;129:203-207.
- Akbar S. Andrographis paniculata: A review of pharmacological activities and clinical effects. Altern Med Rev. 2011;16:66-77.
- Naveed S, Nazeer S, Waheed N. Degradation study of meloxicam by UV-spectroscopy. Br J Res. 2014;1:105-112.
- Brantley SJ, Argikar AA, Lin YS, Nagar S, Paine MF. Herb–drug interactions: Challenges and opportunities for improved predictions. Drug Metab Dispos. 2014;42:301-317.
- Oga EF, Sekine S, Shitara Y, Horie T. Pharmacokinetic herb-drug interactions: insight into mechanisms and consequences. Eur J Drug Metab Pharmacokinet. 2016;41:93-108.
- Zhang X, Zhang X, Wang X, Zhao M. Influence of andrographolide on the pharmacokinetics of warfarin in rats. Pharm Biol. 2018;56:351-356.
- Balap A, Lohidasan S, Sinnathambi A, Mahadik K. Herb-drug interaction of Andrographis paniculata (Nees) extract and andrographolide on pharmacokinetic and pharmacodynamic of naproxen in rats. J Ethnopharmacol. 2017;195:214-221.
- Sinha PK, Jeswani RM, Topagi KS, Damle MC. A validated RP-HPLC method for determination of Meloxicam in the Presence of its Impurities. Int J of Pharm Tech Res. 2009;1:1051-60.
- Emara LH, Emam MF, Taha NF, Raslan HM, El-Ashmawy AA. A simple and sensitive HPLC/UV method for determination of meloxicam in human plasma for bioavailability and bioequivalence studies. J Appl Pharm Sci. 2016;6:012-019.
- Panossian A, Hovhannisyan A, Mamikonyan G, Abrahamian H, Hambardzumyan E, Gabrielian E, et al. Pharmacokinetic and oral bioavailability of andrographolide from Andrographis paniculata fixed combination Kan Jang in rats and human. Phytomedicine. 2000;7:351-364.
- Balap A, Atre B, Lohidasan S, Sinnathambi A, Mahadik K. Pharmacokinetic and pharmacodynamic herb–drug interaction of Andrographis paniculata (Nees) extract and andrographolide with etoricoxib after oral administration in rats. J Ethnopharmacol. 2016;183:9-17.
- Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27.
- Amdekar S, Roy P, Singh V, Kumar A, Singh R, Sharma P. Anti-inflammatory activity of lactobacillus on carrageenan-induced paw edema in male wistar rats. Int J Inflamm. 2012.
- Hafeez A, Jain U, Sajwan P, Srivastava S, Thakur A. Evaluation of Carrageenan induced anti-inflammatory activity of ethanolic extract of bark of Ficus virens Linn., in swiss albino mice. J Phytopharmacol. 2013;2:39-43.
- Goncalves D, Calou IBF, Siqueira RP, Lopes AA, Leal LKA, Brito GAC, et al. In vivo and in vitro anti-inflammatory and anti-nociceptive activities of lovastatin in rodents. Brazilian J Med Biol Res. 2011;44:173-181.
- Mahmood KT, Khan B, Ashraf M, Haq IU. Specific and simple hplc assay of ecofriendly meloxicam in pharmaceutical formulations. J Pharm Sci Res. 2010;2:878.
- Rogatsky, Eduard, Daniel S. Bioanalytical method validation guidance. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1043:25.
- Shen YC, Chen CF, Chiou WF. Andrographolide prevents oxygen radical production by human neutrophils: possible mechanism (s) involved in its anti‐inflammatory effect. Br J Pharmacol. 2002;135:399-406.
- Lee JY. Pharmacodynamic and pharmacokinetic interactions between herbs and western drugs. Orient Pharm Exp Med. 2008;8:207-214.
- Gurley BJ. Pharmacokinetic herb-drug interactions (Part 1): Origins, mechanisms, and the impact of botanical dietary supplements. Planta Medica. 2012;78:1478-1489.
- Brown CM, Reisfeld B, Mayeno AN. Cytochromes P450: A structure-based summary of biotransformations using representative substrates. Drug Metab Rev. 2008;40:1-100.
- Shyam Prasad G, Srisailam K, Sashidhar RB. Metabolic inhibition of meloxicam by specific CYP2C9 inhibitors in Cunninghamella blakesleeana NCIM 687: In silico and In vitro studies. Springer Plus. 2016;5:166.
- Martignoni M, Groothuis GM, De Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2:875-894.
- Samala S, Veeresham C. Pharmacokinetic and pharmacodynamic interaction of boswellic acids and andrographolide with glyburide in diabetic rats including its PK/PD modeling. Phytother Res. 2016;30:496-502.
Citation: Mahadik KR, Srinivasan M, Sathiyanarayanan L, Arulmozhi S (2019) Pharmacokinetic and Pharmacodynamic Interaction of Andrographolide with Meloxicam in Wistar rats. Mod Chem Appl 7:268. doi: 10.35248/2168-975X.19.7.268
Copyright: © 2019 Mahadik KR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


