PMC/PubMed Indexed Articles
Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Scientific Indexing Services (SIS)
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research - (2020) Volume 11, Issue 6
Neutral Surfactant Enhanced Exon-skipping of Morpholino Oligonucleotides in vitro and in mdx Mice
Bo Wu, Drains Morgan, Peijuan Lu, Qilong Lu and Mingxing Wang*Received: 25-Aug-2020 Published: 05-Oct-2020, DOI: 10.35248/2157-7439.20.11.553
Abstract
Antisense oligomers induced exon-skipping has been promising therapy for Duchenne muscular dystrophy in preclinical and clinical trials, but its therapeutic potential could be improved with enhanced delivery approach. A few neutral surfactants were investigated here in for their performance to improve exon-skipping of an antisense phosphorodiamidate morpholino oligomer (PMO) both in vitro and in vivo. This study showed that these surfactants, especially Bigchapand Deoxy Bigchap, improved the delivery efficiency of PMO to the levels comparable to Endoporter-mediated PMO delivery in vitro, and significant enhancement with Deoxy Bigchap-mediated PMO was further observed in mdx mice up to 7-fold compared with PMO alone. Cytotoxicity of the surfactants except for Mega10 was much lower than that by Endoporter in vitro and not clearly observed in vivo under the tested dosage. These results reveal that surfactant’s composition is key factor as delivery carrier to improve PMO exon-skipping efficiency, the efficacy and safety endow neutral surfactants as potential delivering enhancer for oligonucleotide in the treatment of muscular dystrophy or other diseases.
Keywords
Neutral surfactant; Phosphorodiamidate morpholino oligomer; Exon-skipping; Antisense therapy; Muscular dystrophy
Introduction
Antisense therapy is a potential treatment for genetic disorders or infections using synthetic single-stranded DNA-like molecules called antisense oligonucleotides (AOs)which can hybridize to specific targets by Watson-Crick base-pairing rules [1]. Duchenne muscular dystrophy (DMD) is an X-linked, progressive form of muscle degenerative disorder caused by mutations of DMD gene, leading to lack of the dystrophin protein, affecting approximately 1 in 3500-5,000 newborn males [2-5]. AOs have been proven to be a useful therapeutic drug for the DMD treatment by intentionally skipping one or more exonsto restore the reading frame of the mutated transcripts, resulting in the production of truncated, but functional dystrophin proteins [6-18]. Of the antisense oligonucleotides, phosphorodiamidate morpholino oligomer (PMO) has been the most promising macro molecule inducing exon-skipping in the dystrophin gene [7,8,11,19,20]. In particularly, US Food and Drug Administration (FDA) approved EXONDYS 51TM (Eteplirsen) in 2016, which can be applied to 17.5% of DMD populations [21-23], and recently approved VYONDYS 53TM (golodirsen, www.SareptAssist.com) based on promising dada in phase I/II clinical trials [24], demonstrating the potential safety and effectiveness of PMO drug for the treatment of genetic disorders and other diseases. However, the therapeutic potency of this AO drug is inadequate for wide clinical utilization, which is largely attributed to inefficient biodistribution to bodywide muscles upon systemic administration [11]. PMO has nonionic phosphorodiamidate linkages and morpholino rings instead of charged phosphodiester linkage and deoxyribose, respectively, being neutral under physiological condition. These modifications confer its stability in biological system and lower toxicity as compared to other counterparts. Meanwhile, the neutrally charged PMOs is associated with poor cellular uptake or difficulties in crossing biological membranes to reach the cytoplasm, and rapid clearance from systemic circulation, short duration of exonskipping effect requiring high dosage and repetitive administration. These dramatically impede drug’s therapeutic potential [25,26]. To improve the PMOs’ therapeutic efficacy in DMD treatment, chemical conjugations or physical formulations have been explored:1) Cell-penetrating peptides subsequently enriched with arginine directly conjugated to PMO (CPP-PMO) are taken up in vitro by proliferating my oblast and differentiated my tubes via gymnosis, and improved significantly in targeting exon-skipping, making near normal levels of dystrophin expression for whole-body muscles in mdx mice through enhancing cellular uptake of PMO [16,27-35]. 2) Octaguanidium-surfaced dendrimer conjugated PMO (Vivo-PMO) has been tested in mdx mouse model and dystrophic dog model, showing optimized efficiency in splicing modulation and skeletal dystrophin production [14,36,37]. However, the above two conjugates showed higher toxicity from the packed cationic charges, potential peptide-related immune responses, and increased cost due to complicated synthesis and purification procedures preventing them from clinical applications [6,13,14,16,17]. 3) The amphiphilic polymer-mediated delivery approach has been studied by us and ameliorated AOs delivery performance both in vitro and in mdx mice. The amphiphilic nature has been verified as being the key issue of this delivery carrier especially for uncharged PMO [38-41]. 4) Small molecule-aided delivery strategy has also been demonstrated to improve exon-skipping of AOs in mdx mice. Such as, Dantrolene/PMO co-administration increased exon-skipping to restore the mRNA reading frame, and the dystrophin glycoprotein complex in skeletal muscles of mdx mice [42]; Monosaccharideaided AOs demonstrated a glucose-fructose (GF) potentiates PMO activity, restores dystrophin levels in skeletal muscles and achieves functional rescue without detectable toxicity, which is attributed to enhancement of GF-mediated PMO uptake in the muscle [43,44]; Glycine improves PMO potency in mdx mice with marked functional improvement and dystrophin increasement in abdominal muscles compared to PMO alone. Glycine enhances satellite cell proliferation and muscle regeneration by increasing activation of mammalian target of rapamycin complex 1(mTORC1) and replenishing the one-carbon unit pool [45]; Saponins enhances exon-skipping of antisense oligonucleotides delivery in vitro and in mdx mice through improved cellular uptake of AOsdue to Saponin’s amphiphilic nature and restore dystrophin protein in skeletal muscle and cardiac muscles reported by us [46,47]; Aminoglycosides (AG)-formulated AOs studied both in vitro and in vivo indicates that the AG-enhanced PMO delivery is probably attributed to the stable complex and prolonged circulation that are likely resulted from hydrogen-binding between AGs and PMO oligonucleotides, and the inherent conformational adaptability of AG to oligonucleotides [48]. Although some promising results have been obtained by aforementioned studies, to develop efficient and safe delivery approach is still the most challenge to make AOs therapy significantly for the treatment of DMD or other diseases.
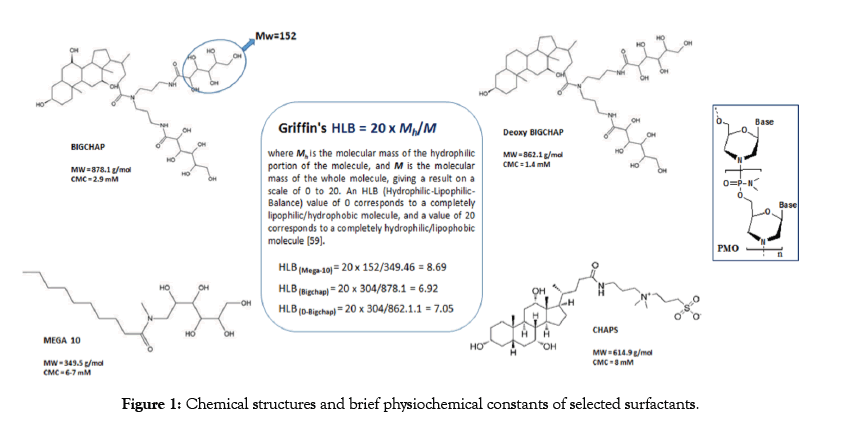
Our previous studies demonstrated that amphiphile with moderate hydrophilic-lipophilic balance (HLB) as delivery carrier is crucial for the enhancement of neutral PMO therapeutic effects, the carrier’s charge is not s oimportant as for negative charged oligonucleotides or pDNA delivery, as well as the polymermediated PMO delivery displaying the more cationic it is, the more toxic it causes both in vitro and in vivo [38-41]. With these factors in mind, we hypothesis that neutral surfactants might improve the PMO delivery outcome through improving cellular uptake by taking advantage of its amphipathic nature, neutral charge and biocompatibility compared with cationic counterparts. Surfactants are amphiphilic molecules that have hydrophobic and hydrophilic parts, typically classified into three kinds based on their polar head:ionic (anionic or cationic), non-ionic (uncharged) or zwitter ionic (having both positively and negatively charged groups, but with a net charge of zero) [49]. Non-ionic surfactants, considered non-denaturing, break lipid-lipid and lipid-protein, but not protein-protein interactions. Therefore, they are widely used in the isolation of membrane proteins in their biologically active form [50,51]. Zwitterionic surfactants have characteristics of both ionic and non-ionic types, especially the steroid-based zwittergents such as CHAPS is less denaturing than linear-chain zwitterionic ones [52]. Neutral surfactants(including non-ionic and zwitterionic)-a kind of amphiphile widely applied in biochemical and medical application ssuch as, diagnostic application, cell culture, membrane protein solubilization, enzymology, antigen/vaccine preparation [52-59]. But less information has been reported about them as drug delivery carrier. We investigated four surfactants based on their molecular structure and potential lower toxicity as demonstrated widely use in biochemistry. They are: Bigchap, Deoxy Bigchap (DBigchap), Chaps and Mega 10 (Figure 1), among them, Bigchap, DBigchap and Mega 10 are non-ionic glucamides, while Chaps belongs to zwitterionic surfactant. Bigchap, DBigchap or Chaps have been used for protein solubilization or adenovirus gene transfer enhancement by taking advantage of their capability to breakdown the glycosaminoglycan (GAG) layer, a hydrophilic polyanionic barrier restricting adenovirus transduction [48,60-64]. The surfactant effects were compared considering their respective parameters, such as composition, HLB, charges, molecular size and critical micelle concentration (CMC). The delivery performances of these surfactants for PMO delivery both in vitro and in vivo of mdx mice, and the interaction between surfactant and PMO were described herein.
Figure 1: Chemical structures and brief physiochemical constants of selected surfactants
Materials and Methods
Materials
Phosphorodiamidate morpholino oligomer (PMO) and End porter were purchased from Gene Tools (Philomath, OR, USA). Surfactants were obtained from G-BIOSCIENCES (St. Louis, MO, USA). 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2- (4-sulfophenyl)-2H-tetrazolium (MTS) was ordered from BioVision Inc (San Francisco, CA, USA).Dulbecco’s modified eagle’s medium (DMEM),4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer solution (HEPES, 1M),L-glutamine, penicillin-streptomycin, fetal bovine serum (FBS),goat anti-rabbit IgG Alexa 594,Lysotracker® Red DND-99 and Hoechst 33342 all were gotten from Thermo Fisher Scientific (Carlsbad, CA, USA). All other chemicals were ordered from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated.
Cell Culture
The C2C12E50 myoblasts and C2C12E23 differentiated cells expressing human dystrophin exon 50 sequence (hDyE50), mouse dystrophin exon 23 sequence (mDyE23) in the GFP coding sequence, respectively, were used in this study. Cells were cultured in 10% FBS-DMEM at 37°C in a humidified 10% CO2 atmosphere. The exon-skipping efficiency was determined by the expression of the reporter GFP [65].
Cytotoxicity study
Cell viability was determined by MTS-based assay. Briefly, C2C12E50 cells were seeded in a 96-well plate (1 x 104/well) in 200 μL 10% FBS-DMEM. The surfactants were added to the cells at various concentrations when the cells reaching to 70% confluence. After 24 hours treatment, 20 μL of Cell Titer 96®Aqueous One Solution Proliferation Kit (MTS solution) was added to each well, and the cells were then incubated for further 4 hours. The cell viability was calculated based on the absorbance measured at 570 nm with Tecan 500 Plate reader (Tecan US, Inc, Morrisville, NC, USA) as previous report [38-41].
Protein adsorption study
Briefly, 50 μL of surfactant solution (1.0 mg/mL) was mixed with 50 μL of bovine serum albumin (BSA) solution (2.0 mg/mL). The surfactant/PMO complex at the ratio of 10. After shaking for 0.5 h at 37°C followed by centrifugation, 50 μL mL of the supernatant was carefully collected, and then the concentration of BSA in the supernatant was monitored with a Nano Drop 2000 spectro photometer (Thermo Fisher Scientific, Waltham, MA, USA) measured absorption at 280 nm. The protein adsorbed on the complexes was calculated as: A% = 100(CiVi-CV)/CiVi. Where Ciand Care the initial BSA concentration and the BSA concentration in the supernatant, respectively; Vi and V are the initial volume of BSA solution and the total volume of BSA after adsorption, respectively [66].
Affinity study between surfactant and PMO
UV-Vis study: The absorbance of surfactant/PMO formulations (1.5 μL) at desired weight ratios was monitored at 260 nm measured with NanoDrop 2000 at room temperature.
Transmission electron microscopy: The surfactants/PMO formulations (1 μg of PMO) at different weight ratios in 100 μL 0.9% saline were prepared and illustrated using negatively stained transmission electron microscopy (TEM; JEM-1400Plus, JEOL USA, Inc.) as previous report [46,48].
In vitro transfection
Cells were seeded 96-well plate (1 x 104/well) in 200 μL 10% FBS-DMEM medium and allowed to grow until 70%confluence. The cell medium was refreshed before addition of surfactant/ PMO formulation with varying ratio (Endoporter was used as positive control). After three or six-day incubation, the transfection efficiencies indicated by levels of GFP production were recorded with the Olympus IX71 fluorescent microscope (Olympus America Inc., Melville, NY, USA). Cells were then harvested, used for quantitative fluorescence-activated cell sorting (FACS) analysis or reverse transcription polymerase chain reaction (RT-PCR) in C2C12E50 and C2C12E23 cell lines, respectively [41,48]. The intensity of the bands was measured with the National Institute of Health (NIH) ImageJ 1.42 (Bethesda, MD, USA) and the percentage of exon-skipping was calculated based on the intensity of the two bands including target exon skipped and unskipped. Product with a skipped band only was considered 100% skipping.
Cellular uptake and intracellular localization
This study was performed and analyzed as reported [41,48]. Briefly, C2C12 cells were seeded onto 12-well plate (5 x 104/ well)and treated with surfactant formulated fluorescence-labeled PMO(FL-PMO) complex at the predetermined ratio for24 hours incubated at 37°C in a humidified 10% CO2 atmosphere. Cells were washed with warm 1xPBS to remove any residual surfactant/ PMO formulations not taken up by cells and incubated with media containing Lysotracker® Red DND-99 to monitor lysosomes for 30 min per manufacturer’s recommendation. Cells were further counterstained with Hoechst 33342 for 15 min, and then imaged with Olympus IX71 fluorescent microscope.
In vivo delivery
All animal experiments in this work were performed in strict accordance with the guidelines approved by the Institutional Animal Care and Use Committee (IACUC), Carolinas Medical Center (Breeding protocol: 10-13-07A; Experimental protocol: 10- 13-08A).
Animals and injections: Five Dystrophic mdx mice (aged 4-5 weeks, C57BL/10 as genetic background) per group(both genders, 3m+2f or 2m+3f) were used. The PMOE23 targeting the boundary sequences of exon and intron 23 of mouse dystrophin gene was used. Surfactant-formulated PMOE23 (2 μg) in 40 μL saline was injected for each Tibialis anterior (TA) muscle, PMO alone as control. The muscles were harvested after two-week treatment, snap-frozen in liquid nitrogen-cooled isopentane and stored at -80oC for later analysis [30-32].
Immunohistochemistry: Serial sections of 6 μm thick were cut from the TA muscles. Sections were stained with a rabbit polyclonal antibody P7 for the detection of dystrophin protein, and further stained by goat anti-rabbit IgG Alexa 594 [13,15,16]. The number of dystrophin-positive fibers in each section was counted by the images taken with the Olympus BX51 fluorescent microscope (Olympus America Inc., Melville, NY, USA).
Statistical analysis
The data were analyzed for statistical significance in using both One-way ANOVA and Student’s t-test with a value of p ≤ 0.05, p ≤ 0.001 being considered significant and very significant difference, respectively. All data are reported as mean ± SD.
Results and Discussions
Cytotoxicity
The in vitro cytotoxicity of these surfactants was determined using MTS-based assay in C2C12E50 cell line with serial concentrations (from 10 μg/mL to 400 μg/mL) as illustrated in Figure 2. The Bigchap, DBigchap and Chaps showed much less toxicity compared with Mega10, which indicated that the molecular targets of these surfactants may be different depending on their hydrophobic part. The higher toxicity of Mega10 against others was probably due to its single short hydrocarbon chain that can insert and translocate faster across lipid bilayer membranes than the amphiphiles bearing longer or bigger ones [67]. The Bighap and DBigchap showed high cell viability close to 90% even at the high dose of 400 μg/ mL, and Chaps were over 80% cell living; whileMega10 gave the cell viability down to below 50% at the dose of 200 μg/mL, though much less toxicity compared with Endoporter (weak-base amphiphilic peptides) showing below 40% at the dose of 20 μg/ mL. This result confirmed our expectation and are in agreement well with our previous polymer-based PMO delivery system, that is the less cationic the compound is, the low toxicity it behaviors [38-41]. In addition, these surfactants except for Mega 10 showed less cytotoxicity compared with Aminoglycosides (AG) and Saponins reported previously [46,48], which should be attributed to their structural property-relatively small molecular size, uncharged amphiphile with moderate LHB. The low cytotoxicity indicates that these surfactants, especially Bigchap, DBigchapor Chaps could be better vehicle candidates for PMO delivery in vitro and in vivo.
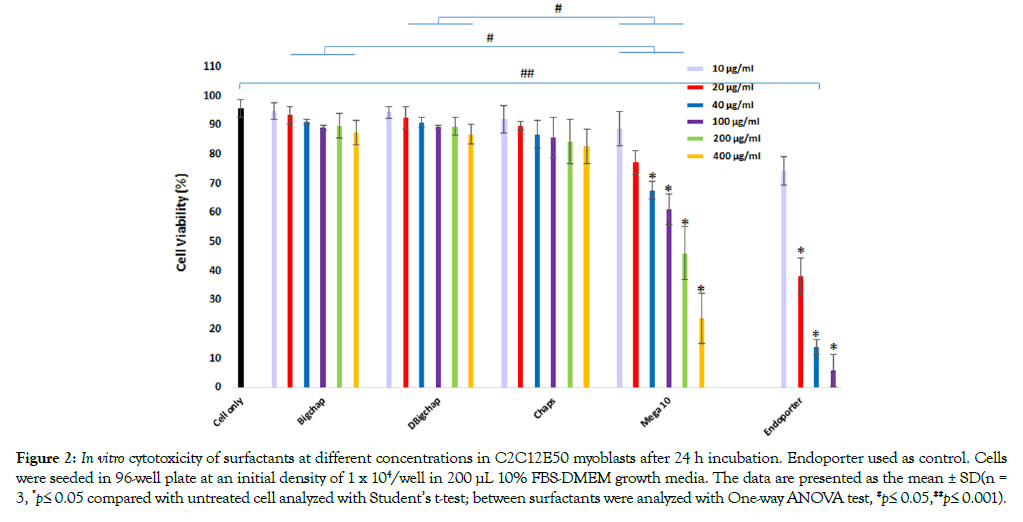
Figure 2: In vitro cytotoxicity of surfactants at different concentrations in C2C12E50 myoblasts after 24 h incubation. Endoporter used as control. Cells were seeded in 96-well plate at an initial density of 1 x 104/well in 200 μL 10% FBS-DMEM growth media. The data are presented as the mean ± SD(n = 3, *p≤ 0.05 compared with untreated cell analyzed with Student’s t-test; between surfactants were analyzed with One-way ANOVA test, #p≤ 0.05,##p≤ 0.001).
Protein adsorption study
Proteins adsorbed onto the surface of biomaterials are known to be able to trigger thrombosis and blood coagulation, which can cause failure in organ function with life-threatening consequence. In system circulation, serum proteins could bind to complexes in a non-specific manner, leading to aggregation and severely decreased TE. The protein adsorption assay of the surfactant was performed here to evaluate their serum tolerance. As shown in Figure 3A, the BSA adsorptions were 37.6%, 39.7%, 42.1%, 38.3%, and 70.1% for Bigchap, DBigchap, Chaps, Mega10 and Endoporter, respectively.
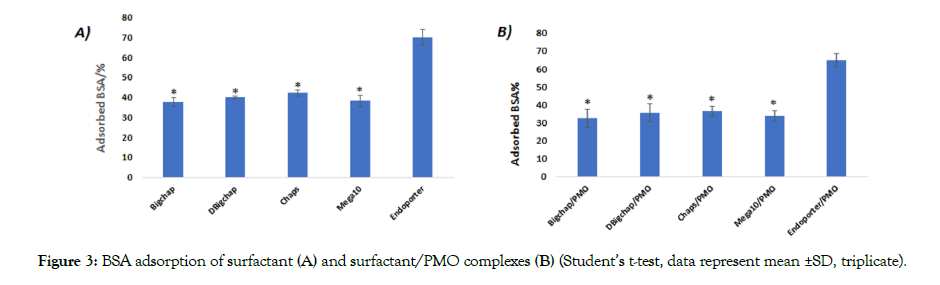
Figure 3: BSA adsorption of surfactant (A) and surfactant/PMO complexes (B) (Student’s t-test, data represent mean ±SD, triplicate).
The Endoporter’s higher adsorption to BSA is probably due to its’ cationic nature and larger molecular size compared to these four surfactants. However, no remarkable differences were observed among these surfactants with BSA adsorption, even the zwitter ionic Chaps. Similarly, as shown in Figure 3B, the complexes formed by surfactant/PMO exhibited much lower protein absorption than that formed by Endoporter/PMO formulation. This result demonstrated the relatively weak interaction between neutral surfactant and BSA, thus preventing their aggregation with negatively charged serum proteins. Furthermore, confirming the strong BSA absorption with Endoporter was primarily caused by additional electrostatic interaction between negatively charged BSA and the positively charged delivery carrier. The good tolerance to serum indicates the neutral surfactant’s advantage as delivery carrier for drug/gene delivery.
Interaction between surfactant and PMO
The affinity between carrier and oligonucleotides is very important for the formation of appropriate particle size, density and surface charge and, consequently, the stability of complex, their uptake and release, ultimately to delivery efficiency. We examined the interactions between the surfactant and PMO at different weight ratios by UV-Vis spectrum, Bigchap/PMO complexes at the weight ratios from 1:1 to 40:1 were examined here (Figure 4A). The Bigchap/PMO complex displayed hyperchromic effect compared with PMO only at all ratios from 1:1 to 40:1, while the absorbance increased from 1:1 to 10:1, and then decrease as the ratio increasing from 20:1 to 40:1.This result indicates that Bigchap might cover PMO completely and shield the PMO’s absorbance between 10:1 and 20:1, the Bigchap/PMO complex compacted strongly through hydrophobic interaction and H-bondsto form suitable conformation. In addition, we investigated all four surfactant/PMO complexes at same ratio of 20:1 (Figure 4B). The data showed that Bigchap, Chaps and Mega10 all three with PMO complexes had hyperchromic effect, but DBigchap/PMO complex shows similar or a little hypochromic effect against PMO alone, showing very distinct from other three complexes, though only one hydroxy group differs between Bigchap and DBigchap, which means DBigchap/PMO probably could not form solid particles or aggregation at this ratio (these four surfactants alone no absorbance at 260 nm). The result indicates that these neutral surfactants have similar affinity with PMO (no chromic shift observed) due to their shared amphiphilic nature and similar composition, although different in structure. Furthermore, we assessed all these surfactants/PMO complexes at the weight ratio of 20:1, and Bigchap/PMO at the ratios from 1:1 to 20:1, as well as surfactants alone under TEM analysis. As illustrated in Figure 5, PMO oligonucleotides alone formed particles with the sizes between 10-30 nm, probably because of hydrophobic interaction and hydrogen-bond among PMO molecules. Bigchap and Chaps alone showed similar particle size and bigger than Mega10, this probably is relevant to their difference in molecular size and structure. The complex of Bigchap/PMO or Chaps/PMO also shaped similarly with the size of nanoparticles slightly bigger compared to Mega10/ PMO complex (5-15 nm), this is likely due to Mega10’s less steric hinderance resulted from its single long-chain and small molecular size compared with other three molecules. However, DBigchap/PMO complex demonstrated larger particle size as compared to the other three complexes under the same ratio of 20:1,probably due to aggregation or less hydrogen-bond between DBigchap and PMO, which is in concordance with the UV-Vis result. On the other hand, Bigchap/PMO complex’s particle size were decreased as the ratio increasing from 1:1(20-40 nm) to 10:1 (5-15 nm) and then increase again from 10:1 to 20:1 (10-30 nm), indicating that Bigchap/PMO particles reached maximum in density between the ratio 10:1-20:1, which is also in agreement well with the above UVVis display. Clearly, the mechanisms of interaction between PMO and the neutral surfactant remain to be more clarified for further improvement of delivery efficiency.
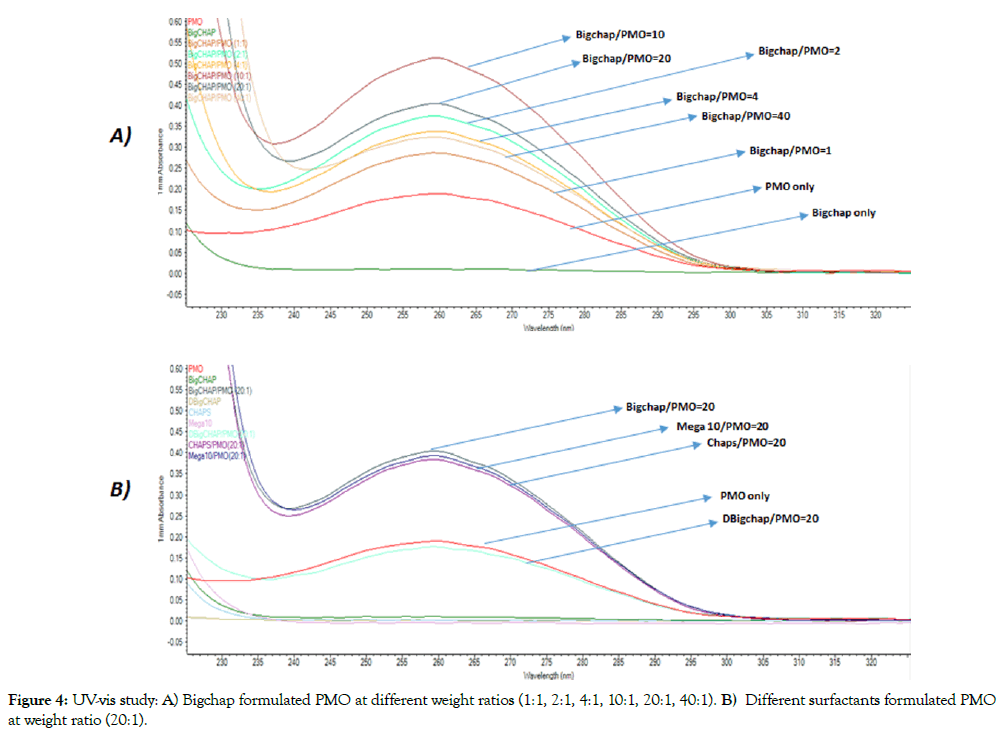
Figure 4: UV-vis study: A) Bigchap formulated PMO at different weight ratios (1:1, 2:1, 4:1, 10:1, 20:1, 40:1). B) Different surfactants formulated PMO at weight ratio (20:1).
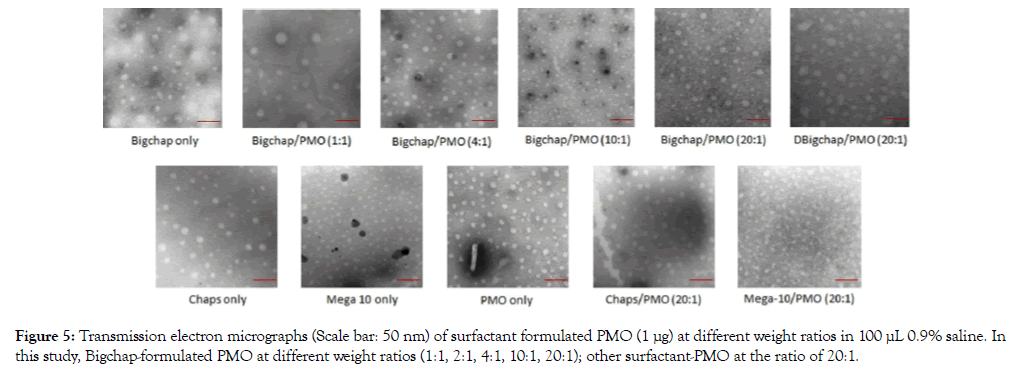
Figure 5: Transmission electron micrographs (Scale bar: 50 nm) of surfactant formulated PMO (1 μg) at different weight ratios in 100 μL 0.9% saline. In this study, Bigchap-formulated PMO at different weight ratios (1:1, 2:1, 4:1, 10:1, 20:1); other surfactant-PMO at the ratio of 20:1.
Delivery in vitro
Firstly, the C2C12E50 myoblasts were used to evaluate the efficacy of surfactantsfor the delivery of PMOE50(5'- AACTTCCTCTTTAACAGAAAAGCATAC-3') [65].In view of above cytotoxicity, affinity study results, the cells were treated with surfactant-formulated PMO (PMOfixed at 10 μg/mL) at six escalating doses (10, 20, 40, 100, 200 and 400 μg/mL) in 200 μL 10% FBS-DMEM medium.To visualize the transfection effect, the GFP expression in C2C12E50 cells was observed with an inverted fluorescent microscope. Figure 6A presents some typical fluorescence images of C2C12E50 cells transfected by surfactant/ PMO formulation at different ratios. The strong fluorescence could be observed when the transfections were mediated by surfactants/ PMO, such as Bigchap, DBigchap and Chaps, especially at higher dosages (100, 200 and 400μg/mL). This efficiency is better than or close to the one achieved by Endorpoter (as positive control), and clearly dose-dependent shown with Bigchap or DBigchap. However, the expression could hardly be detected when the transfection was mediated by naked PMO (as negative control). The results were further quantitively evaluated with the flow cytometry (Figure 6B) and demonstrated that Bigchap, DBigchap and Chaps significantly improved GFP expression at the dose of 40 μg/mL compared with PMO alone. Particularly, Bigchapa nd DBigchap achieved the remarkable transfection level of 86% and 76% at 400 μg/mL, respectively, higher than or comparable to the 83% achieved by Endoporter at the optimum dose of 10 μg/mL. More importantly, toxicity of these two surfactants formulated PMO indicted by over 83% alive cells at the dose up to 400 μg/mL was much lower than that with Endoporter. While, Chaps/PMO reached around 50% transfection, accompanied by cell viability dropped to 58%; Mega10/PMO showed a lower transfection efficiency with higher toxicity as compared to other three surfactants/PMO formulation, although the cytotoxicity remained lower than Endoporter/PMO. The cytotoxicity of surfactant/PMO formulation showed similar trend as surfactant only, confirming the PMO being at safe dosage. The low efficacy and higher cytotoxicity of Mega10 were probably resulted from its smaller molecule and single-long hydrophobic chain , whereas higher efficacy and lower toxicity of Bigchap, DBigchap or Chaps were probably resulted from higher molecular size and better cell-permeability from hydrophobic part. The levels of GFP expression were dominantly surfactant’s structuredependent: the more hydrophobic and /or larger molecule they are, the more effective as PMO delivery vector, which is in line well with our previous studies [38-41]. The smaller and relatively higher hydrophilic Mega10 (Mw = 349.5 g/mol, HLB = 8.69) showed much less efficiency (below 20 % GFP expressed at the optimum dose of 100 μg/mL) compared with other three: Bigchap (Mw = 878.1 g/mol, HLB = 6.92), DBigchap (Mw = 862.1 g/mol, HLB = 7.05), and Chaps (Mw = 614.9 g/mol). The produced GFP from Bigchap, DBigchap, or Chaps-mediated PMO delivery was about 16, 14, and 8-fold higher compared with that of PMO alone at its optimum dosage, respectively. In addition, the exonskipping efficacy was dose-dependent, especially, Bigchap and DBigchap gave improved efficacy significantly as the dosage increasing, while Mega10 showed lower cell viability and GFP expression after achieving the peak delivery rate owing to its higher cytotoxicity. The Bigchap and DBigchap gave higher transfection performances compared with Aminoglycosides, and better than or close to Digitonin (one of Saponins) for PMO delivery at the optimum condition [46-48]. Saponins can improve delivery not only for neutral PMO, but for negatively charged oligonucleotides also [46,47]; while, Aminoglycosides and these neutral surfactants can only work for PMO [48]. This is probably relevant to their structure nature and different mechanism to enhance delivery efficiency for drug or gene, though the mechanism remains to be clarified further. In addition, generally, the small molecules help oligonucleotides delivery at wider effective dosage window (40-400 μg/ml) as compared to polymers we developed previously (10-100 μg/ml) due to low toxicity. These data support the hypothesis that the larger or more hydrophobic the molecule is, the more effective it conducts as drug/gene delivery carrier, while, higher toxicity could also narrow dosage window limiting clinical application [38-41]. The result indicated that Bigchap and DBigchap have clearly higher potential as PMO delivery enhancer probably as a result of their remarkable transduction, good serum tolerance, biocompatibility and low cytotoxicity.
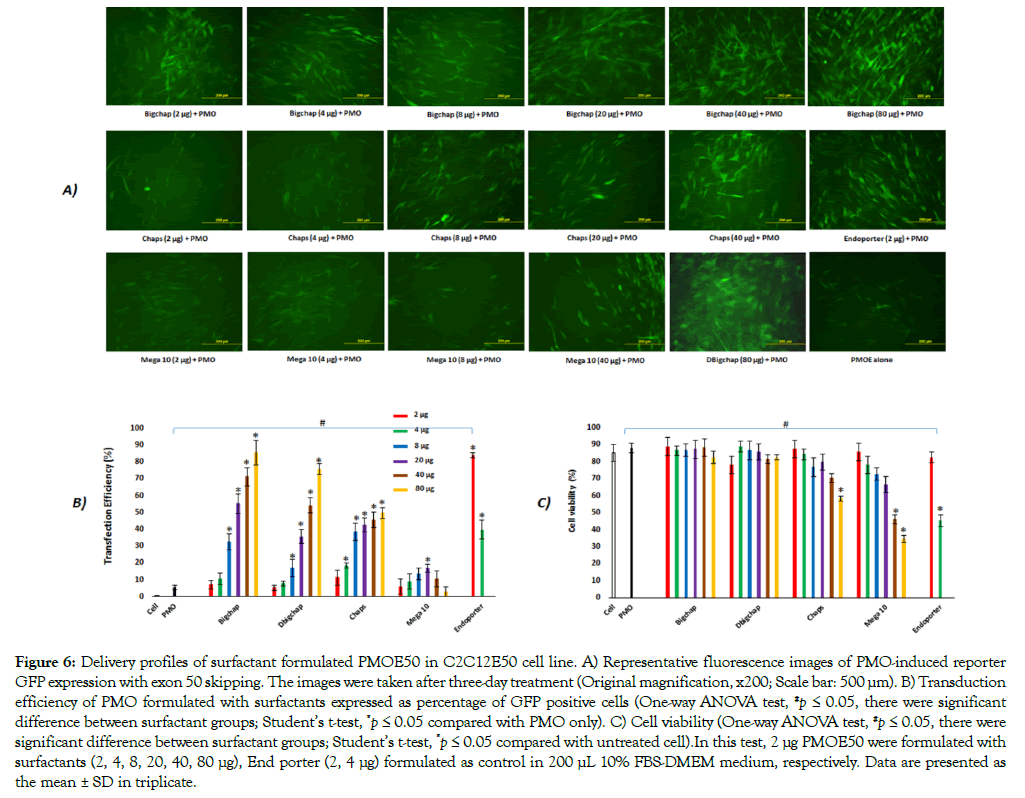
Figure 6: Delivery profiles of surfactant formulated PMOE50 in C2C12E50 cell line. A) Representative fluorescence images of PMO-induced reporter GFP expression with exon 50 skipping. The images were taken after three-day treatment (Original magnification, x200; Scale bar: 500 μm). B) Transduction efficiency of PMO formulated with surfactants expressed as percentage of GFP positive cells (One-way ANOVA test, #p ≤ 0.05, there were significant difference between surfactant groups; Student’s t-test, *p ≤ 0.05 compared with PMO only). C) Cell viability (One-way ANOVA test, #p ≤ 0.05, there were significant difference between surfactant groups; Student’s t-test, *p ≤ 0.05 compared with untreated cell).In this test, 2 μg PMOE50 were formulated with surfactants (2, 4, 8, 20, 40, 80 μg), End porter (2, 4 μg) formulated as control in 200 μL 10% FBS-DMEM medium, respectively. Data are presented as the mean ± SD in triplicate.
These surfactants for PMO delivery were further tested in C2C12E23 cell line expressing GFP containing mDyE23 and induced to differentiate to myotubes [65]. The cells were treated with surfactant formulated PMOE23(5'- GGCCAAACCTCGGCTTACCTGAAAT-3'). In view of the results from PMOE50 delivery, sixdosages (10, 20, 40, 100, 200 and 400 μg/mL) of each surfactant complexed with PMO (10 μg/ mL) in 200 μL 10% FBS-DMEM medium were tested (Figure 7). Higher levels of GFP expression and exon 23 skipping with Bigchap, DBigchap and Chaps were observed by fluorescence images and RT-PCR against PMO alone. Again, the exon-skipping efficiency with Bigchap and DBigchap was higher than or comparable to Endoporter-mediated PMO delivery, which is accordance well with that achieved in PMOE50. The E23 skipping-levels were around 100%, 55.1%, 65.9%, 48.5% and 53.7% with Bigchap (40 μg), DBigchap (40 μg), Chaps (40 μg), Mega 10 (40 μg) and Endoporter (2 μg)-formulated PMOs, respectively. The skippinglevels of dose-dependent observed were 58.7% (2 μg), 65.3% (4 μg), 73.8% (8 μg), ≈100% (40 μg) with Bigchapin comparison of 29.6% with PMO only. These data demonstrated that surfactant could advance delivery of PMO not only for C2C12E50 myoblast, but for C2C12E23 differentiated myotubes also, indicating the potential for in vivo study.
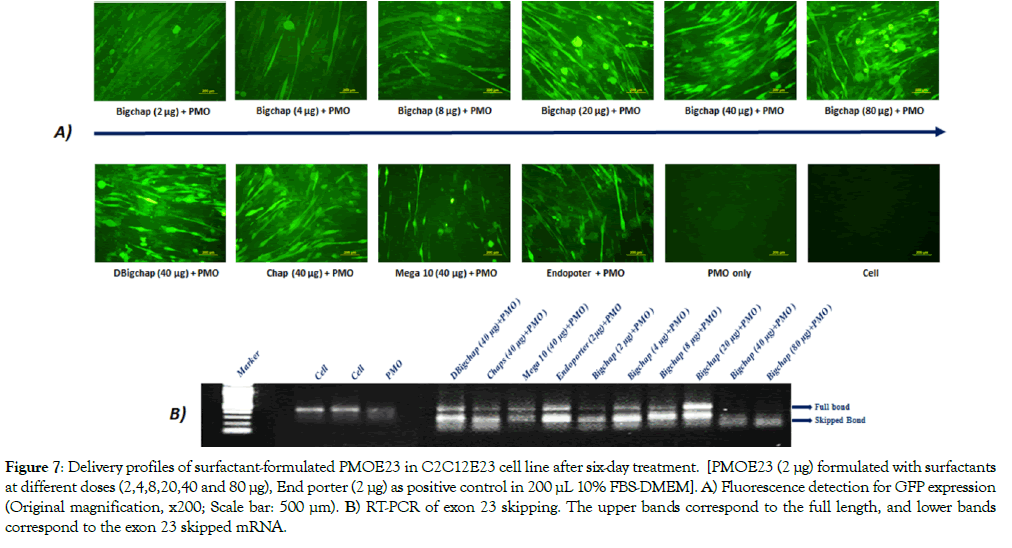
Figure 7: Delivery profiles of surfactant-formulated PMOE23 in C2C12E23 cell line after six-day treatment. [PMOE23 (2 μg) formulated with surfactants at different doses (2,4,8,20,40 and 80 μg), End porter (2 μg) as positive control in 200 μL 10% FBS-DMEM]. A) Fluorescence detection for GFP expression (Original magnification, x200; Scale bar: 500 μm). B) RT-PCR of exon 23 skipping. The upper bands correspond to the full length, and lower bands correspond to the exon 23 skipped mRNA.
Cellular uptake and Intracellular localization
To better understand the improved PMO delivery efficacy with the aid of surfactant in cell, we studied the cell uptake behavior and intracellular localization of surfactant/PMO complex, Bigchap was complexed with FL-PMO at a weight ratio of 5/1. As expected, the presence of Bigchap apparently improved the cellular uptake of oligonucleotide PMO as demonstrated by stronger green fluorescence which localized with the lysosomal marker ,and some PMO also enter the nuclei of C2C12 cells monitored by over laid signals (yellow or white-yellow signals in merged one) stronger than that of PMO alone (Figure 8). This result supports the ability of Bigchap to enhance the delivery of oligonucleotide by increasing the efficiency of PMO crossing membrane lipid bilayer to reach the cytoplasm.
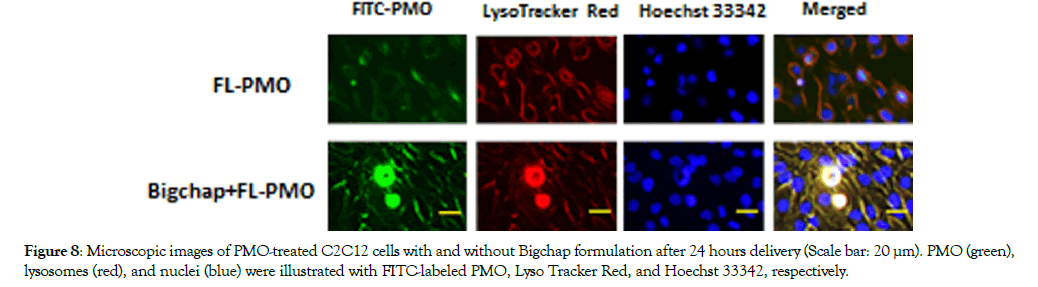
Figure 8: Microscopic images of PMO-treated C2C12 cells with and without Bigchap formulation after 24 hours delivery (Scale bar: 20 μm). PMO (green), lysosomes (red), and nuclei (blue) were illustrated with FITC-labeled PMO, Lyso Tracker Red, and Hoechst 33342, respectively.
Delivery in vivo
Furthermore, the effect of the surfactants for PMO delivery in vivo was evaluated in mdx mice-containing a nonsense mutation in the exon 23, preventing the production of functional dystrophin protein. PMO targeting dystrophin exon 23 injected to Tibialis anterior (TA) muscle can remove the mutated exon 23 and restore the reading-frame of dystrophin transcripts, leading to expression of a truncated but in-frame dystrophin protein [13,15,16].According to the in vitro performance and affinity study between surfactants and PMO, we chose 20 μg surfactant as effective, safe dosage and formulated with PMOE23 (2 μg) for test by intramuscular (i.m.) injection.
Immunohistochemistry indicated that the surfactant-formulated PMOE23 dramatically increased the numbers of the dystrophinpositive fibers in the treated muscles being up to53%, 73%, 57%, and 35% in one cross section of the TA muscle for Bigchap, DBigchap, Chaps and Mega10 formulated PMOs, respectively (Figure 9). DBigchap mediated one was especially effective with dystrophin positive fibersover 7-foldof that achieved with PMOalone (≈10%). The highest enhancement for PMO delivery achieved by DBigchap in vivo, is probably attributed to its lowest CMC (1.4 mM) compared others (Bigchap, 2.9 mM; Chaps, 8 mM; Mega10, 6-7 mM). The lower the CMC of micelle is, the lower dose used to prepare a particle formulation in order to counter the dilution effects for the sustained release in the blood. The outcome does not correlate very well with the data in vitro, indicating the difficulty to predict in vivo performance based on in vitro behavior, and further confirming that the composition of the vector’s molecular structure is crucial to improve the delivery efficiency of therapeutic agent both in vitro and in vivo as previously reported. Particularly, considering overall performances including cytotoxicity and delivery efficiency, the DBigchap-PMO formulation is very promising compared with Saponins [46] or Aminoglycoside [48] and Endoporter or other polymers [38-42]. In addition, this amphiphile enhancer provides flexible delivery approach for PMO in vivo compared with other small molecule-added delivery approaches [42-45],typically for the treatment of DMD-long-term and systemic administration needed. These results demonstrated that: 1) Neutral surfactant has great potential for PMO delivery, indicating that the delivery carrier’s charge is not so important for uncharged PMO as for negatively charged oligonucleotides that requires cationic carrier through electrostatic interaction to stable the complex, and promote internalization into the cells, by means of a good affinity for the cell membranes, especially in vivo microenvironment. 2) The amphiphilic nature of surfactant is essential to improve the delivery efficiency of uncharged PMO through enhanced cellular uptake.3) The hydrophobic interaction and potential H-bonds formed between carrier and PMO are likely capable to condense and stabilize the carrier/PMO complex due to more hydrophobicity of PMO compared with other counterparts, thus improving the delivery efficiency. 4) The better tolerance to serum compared with Endoporter makes surfactant/PMO complex more stable with less off-target effect. 5) The CMC is vital, the lower CMC of the carrier, the lower dosage is likely needed for effectives stained delivery. Taken as a whole, these results further highlight the intricacy between carrier and the delivery cargo, and corresponding deliver performance, demonstrating the potential of neutral surfactants as delivery carrier for PMO both in vitro and in vivo.
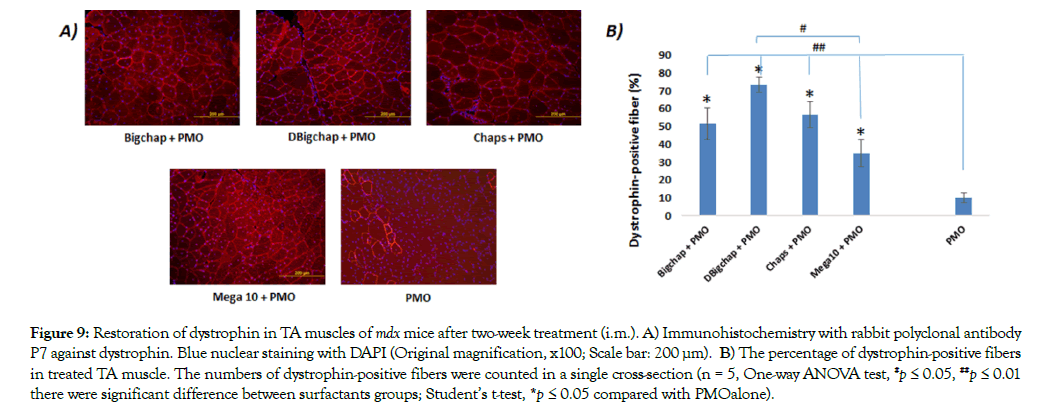
Figure 9: Restoration of dystrophin in TA muscles of mdx mice after two-week treatment (i.m.). A) Immunohistochemistry with rabbit polyclonal antibody P7 against dystrophin. Blue nuclear staining with DAPI (Original magnification, x100; Scale bar: 200 μm). B) The percentage of dystrophin-positive fibers in treated TA muscle. The numbers of dystrophin-positive fibers were counted in a single cross-section (n = 5, One-way ANOVA test, #p ≤ 0.05, ##p ≤ 0.01 there were significant difference between surfactants groups; Student’s t-test, *p ≤ 0.05 compared with PMOalone).
Conclusions
In this study, we investigated four neutral surfactants as delivery carrier for PMO antisense oligonucleotides both in vitro and in vivo in dystrophic mdx mice as well as and interaction for the first time. The results demonstrate that these neutral surfactants, especially Bigchap and DBigchap elevated delivery and achieved efficiency of PMO higher than or comparable to Endoporter-mediated PMO in vitro; the DBigchap-formulated PMO significantly displayed up to 7-fold enhancement over PMO only, and no detectable toxicity observed at the tested dosage in vivo. Overall, this study revealed an alternative approach to deliver PMO effectively and safely, and the data suggest that optimization of surfactants in molecular size and component has potential to further improve the delivery performance of oligonucleotides for the treatment of DMD and other disease.
Acknowledgments
The work was financial supported by the Carolinas Muscular Dystrophy Research Endowment at the Carolinas HealthCare Foundation and Carolinas Medical Center, Charlotte, NC. The authors thanks Dr. Fei Guo and Dr. David M Foureau for their technical assistance in Flow cytometry study; Mrs. Daisy M. Ridings, Stephanie Williams and Anthony Dart for TEM measurement.
Authors’ Contributions
M.W. conceived, designed, performed the whole experiments except for in vivo part, and wrote the paper; B.W. supervised and performed the in vivo experiments with D.M.; P.L. contributed in cell culture; Q.L., and B.W. reviewed the manuscript.
Conflict of Interest
There are no conflicts to declare.
REFERENCES
- Lee JJA, Yokota T. Antisense Therapy in Neurology. J Pers Med 3:144-176.
- Hoffman EP, Brown RHJr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1958; 51: 919-928.
- Emery AE. The muscular dystrophies. Lancet 2002; 359: 687-695.
- Wagner KR, Lechtzin N, Judge DP. Current treatment of adult Duchenne muscular dystrophy. Biochim Biophys Acta 2007; 772:229-237.
- Mendell JR, Shilling C, Leslie ND, Flanigan KM, al-Dahhak R. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neuro 2012; l71: 304-313.
- Amantana A, Moulton HM, Cate ML, Reddy MT. Pharmacokinetics, biodistribution, stability and toxicity of a cell-penetrating peptide-morpholino oligomer conjugate. Bioconjugate Chem 2007; 18(4):1325-1331.
- Goemans NM, Tulinius M, van den Akker JT, Burm BE. Systemic administration of PRO051 in Duchenne’s muscular dystrophy. N Engl J Med 2012; 364:1513-1522.
- Kinali M, Arechavala-Gomeza V, Feng L, Cirak S. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neuro 2009; l8: 918-928.
- Lu Q, Mann CJ, Lou F Bou-Gharios G, Morris GE. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nat Med 2003; 9(8):1009-1014.
- Lu Q, Rabinowitz A, Chen Y, Yokota T. Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proc Natl Acad Sci 2005; 102(1): 198-203.
- MendellJ R, Rodino-Klapac LR, Sahenk Z, Roush K, Bird L. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann Neuro 2013; l74(5): 637-647.
- van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med 2007; 357:2677-2686.
- Wu B, Moulton HM, Iversen PL, Jiang J. Effective rescue of dystrophin improves cardiac function in dystrophin-deficient mice by a modified morpholino oligomer. Proc Natl Acad Sci 2008;105: 14814-14819.
- Wu B, Li Y, Morcos PA, Doran TJ. Octa-guanidine morpholino restores dystrophin expression in cardiac and skeletal muscles and ameliorates pathology in dystrophic mdx mice. Mol Ther 2009; 17: 864-871.
- Wu B, Lu P, Benrashid E, Malik S. Dose-dependent restoration of dystrophin expression in cardiac muscle of dystrophic mice by systemically delivered morpholino. Gene Ther 2010; 17:132-140.
- Wu B, Cloer C, Shaban M, Moulton H. Long-term rescue of dystrophin expression and improvement in muscle pathology and function in dystrophic mdx mice by peptide-conjugated morpholino. Am J Pathol 2015; 181(2): 392-400.
- Yin H, Moulton HM, Seow Y, Boyd C. Cell-penetrating peptide-conjugated antisense oligonucleotides restore systemic muscle and cardiac dystrophin expression and function. Hum Mol Genet 2012; 17:3909-3918.
- Evers MM, Toonen LJA, van Roon-Mom WMC. Antisense oligonucleotides in therapy for neurodegenerative disorders. Adv Drug Del Rev 2015; 87:90-103.
- Cirak S, Arechavala-Gomeza V, Guglieri M, Feng L. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet 2011; 378(9791): 595-605.
- Malerba A, Sharp PS, Graham IR, Arechavala-Gomeza V. Chronic systemic therapy with low-dose morpholino oligomers ameliorates the pathology and normalizes locomotor behavior in mdx mice. Mol Ther 2011; 19: 345-354.
- Aartsma-Rus A, Krieg AM. FDA Approves eteplirsen for Duchenne muscular dystrophy: the next chapter in the eteplirsen saga. Nucleic Acid Ther 2017; 27:1–3.
- Kesselheim AS, Avorn J. Approving a problematic muscular dystrophy drug. JAMA 2016; 316:2357.
- Stein CA. Eteplirsen approved for duchenne muscular dystrophy: the FDA faces a difficult choice. Mol Ther 2016; 24:1884–1885.
- Muntoni F, Frank D, Sardone V, Morgan J. Golodirsen induces exon skipping leading to sarcolemmal dystrophin expression in duchenne muscular dystrophy patients with mutations amenable to Exon 53 skipping (S22.001). Neurology 2018; 90.
- Summerton J, Weller D. Morphoolino antisense oligomers design, preparation, and properties. Antisense Nucleic. Acid Drug Dev 2017; 7:187-195.
- Lu Q, Yokota T, Takeda S, Garcia L. The Status of Exon Skipping as a Therapeutic Approach to Duchenne Muscular Dystrophy. Mol Ther 2011; 19(1):9-15.
- Yin H, Saleh AF, Betts C, Camelliti P. Pip5 transduction peptides direct high efficiency oligonucleotide-mediated dystrophin exon skipping in heart and phenotypic correction in mdx mice. Mol Ther 2011; 19: 1295-1303.
- Lehto T, Alvarez AC, Gauck S, Gait MJ. Cellular trafficking determines the exon skipping activity of Pip6a-PMO in mdx skeletal and cardiac muscle cells. Nucleic Acids Res 2014; 42(5):3207-3217.
- Wu B, Cloer C, Lu P, Milazi S, Shaban M. Exon skipping restores dystrophin expression, but fails to prevent disease progression in later stage dystrophic dkomice. Gene Ther 2014; 21:785-793.
- Yin H, Moulton HM, Betts C, Seow Y, Boutilier J. A fusion peptide directs enhanced systemic dystrophin exon skipping and functional restoration in dystrophin-deficient mdx mice. Hum Mol Genet 2009;18:4405-4414.
- Xianjun G, Jingwen Z, Dong X, Cao L. Effective dystrophin restoration by a novel musclehoming peptide-morpholino conjugate in dystrophin-deficient mdx mice. Mol Ther 2014; 22:1333-1341.
- Betts C, Saleh AF, Arzumanov AA, Hammond SM, Godfrey C. Pip6-PMO, a new generation of peptideoligonucleotide conjugates with improved cardiac exon skipping activity for DMD treatment. MolTher- Nucleic Acids 2012; 1(8):e38.
- Betts CA, Saleh AF, Carr CA, Hammond SM. Prevention of exercised induced cardiomyopathy following Pip-PMO treatment in dystrophic mdx mice. Sci Rep 5: 8986.
- EchigoyaY, Nakamura A, Nagata T, Urasawa N, Lim KRQ. Effects of systemic multiexon skipping with peptide-conjugated morpholinos in the heart of a dog model of Duchenne muscular dystrophy. Proc Natl Acad Sci USA 2017; 114: 4213-4218.
- Jearawiriyapaisarn N, Moulton HM, Sazani P, Kole R. Long-term improvement in mdx cardiomyopathy after therapy with peptide-conjugated morpholino oligomers. Cardiovasc Res 2010; 5: 444-453.
- YokotaT, NakamuraA, NagataT and SaitoT. Extensive and prolonged restoration of dystrophin expressionwith vivo-morpholino-mediatedmultiple exon skipping in dystrophic dogs. Nucleic Acid Ther 2012;22:306-315.
- Echigoya Y, Aoki Y, Miskew B, Panesar D. Long-term efficacy of systemicmultiexon skipping targeting Dystrophin exons 45-55 with a cocktail of vivo-morpholinos in Mdx52 mice. MolTher- Nucleic Acids 2015; 4(2):e225.
- Wang M, Wu B, Lu P, Tucker JD. Polyethylenimine-modified Pluronics (PCMs) Improve Morpholino Oligomer Delivery in Cell Culture and Dystrophic mdx Mice. Mol Ther 2013;21: 210-216.
- Wang M, Wu B, Tucker JD, Lu P. Evaluation of Tris[2-(Acryloyloxy)Ethyl]Isocyanurate Cross-Linked Polyethylenimine as Antisense Morpholino Oligomer Delivery Vehicle in Cell Culture and Dystrophic mdx Mice. HumGene Ther 2014; 25(5): 419-427.
- Wang M, Wu B, Tucker JD, Lu P, Bollinger. Tween 85 grafted PEIs enhanced delivery of antisense 2′-O-methyl phosphorothioate oligonucleotides in vitro and in dystrophic mdx mice. J Mater Chem 2015; B3: 5330-5340.
- Wang M, Wu B, Tucker JD, Lu P, Lu, Q. Cationic Polyelectrolyte-mediated Delivery of Antisense Morpholino Oligonucleotides for Exon-skipping in vitro and in mdx Mice. InterJ Nanomed 2015; 10: 5635-5646.
- Kendall GC, Mokhonova EI, Moran M, Sejbuk NE. Dantrolene Enhances Antisense-Mediated Exon Skipping in Human and Mouse Models of Duchenne Muscular Dystrophy. Sci Transl Med 2012; 164(4):164ra160.
- Cao L, Han G, Lin C, Gu B. Fructose Promotes Uptake and Activity of Oligonucleotides with Different Chemistries in a Context-dependent Manner in mdx Mice. MoleTher - Nucleic Acids 2016; 5:e329.
- Han G, Gu B, Cao L, Gao X. Hexose enhances oligonucleotide delivery and exon skipping in dystrophin-deficient mdx mice. Nat Commun 2016; 7:10981.
- Lin C, Han G, Ning H, Song J, Ran N. Glycine Enhances Satellite Cell Proliferation, Cell Transplantation, and Oligonucleotide Efficacy in Dystrophic Muscle. Mol Ther 2020; 28 (5):1339-1358.
- Wang M, Wu B, Shah SN, Lu P. Saponins as Natural Adjuvant for Antisense Morpholino Oligonucleotides Delivery In Vitro and in mdx Mice. MolTher - Nucleic Acids 2018; 11:192-202.
- Wang M, Wu B, Shah SN, Lu P. Saponins enhance exon skipping of 2′-O-methyl phosphorothioate oligonucleotide in vitro and in vivo. Drug Design, Development and Therapy 2018; 12: 3705-3715.
- Wang M, Wu B, Shah SN, Lu P. Aminoglycoside Enhances the Delivery of Antisense Morpholino Oligonucleotides in vitro and in mdx Mice. MolTher - Nucleic Acids 2019; 16: 663-674.
- Sekhon BS. Surfactants: Pharmaceutical and Medicinal Aspects. Journal of Pharmaceutical Technology, Research and Management 2013; 1: 43-68.
- le Maire M, Champeil P, Moller JV. Interaction of membrane proteins and lipids with solubilizing detergents. Biochim Biophys Acta 2004; 1508(1-2): 86-111.
- Seddon AM, Curnow P, Booth PJ. Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta 2004;1666(1-2):105-117.
- Bhairi SM, Mohan C. Detergents. EMD Biosciences, La Jolla, CA.
- Uchegbu IF, Vyas SP. Non-ionic surfactant-based vesicles (niosomes) in drug delivery. InterJ Pharm172: 33-70.
- Jahan K, Balzer S, Mosto P. Toxicity of nonionic surfactants. Environmental Toxicology II281-289. WIT Transactions on Ecology and the Environment WIT Press 2008; 110.
- Shah SK, Bhattaral A, Chatterjee SK. Application of surfactants in modern science and technology. Modern Trends in Science and Technology. Editors: Devendra Adhikari, Shiva Kumar Rai and Kul Prasad Limbu. Publishers: Nepal Biological Society, Nepal Physical Society (Eastern Chapter) and Research Council of Science and Technology 2011; 147-158.
- Kumarn GP, Rajeshwarrao P. Nonionic surfactant vesicular systems for effective drug delivery—an overview. Acta PharmaceuticaSinica 2011; B1(4): 208-219.
- Lorenz-Fonfria V, Peralvarez-Marin A, Padros, Lazarova T. Solubilization, Purification, and Characterization of Integral Membrane Proteins, in Production of Membrane Proteins: Strategies for Expression and Isolation. 1st ed. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany 2013.
- Lorch M, Batchelor R. Stabilizing Membrane Proteins in Detergent and Lipid Systems, in Production of Membrane Proteins: Strategies for Expression and Isolation. 1st ed. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany 2011.
- GriffinWC. (1954). Calculation of HLB Values of Non-Ionic Surfactants. Journal of the Society of Cosmetic Chemists5(4):249-256.
- Bonelli FS, Jones A. Reaction of lecithin: cholesterol acyltransferase with a water-soluble substrate: effects of surfactants. Biochim Biophys Acta 1993; 1166:92-98.
- Womack MD, Kendall DA, MacDonald RC. Detergent effects on enzyme activity and solubilization of lipid bilayer membranes. Biochim Biophys Acta 1983; 733: 210-215.
- Hjelmeland LM (1980) A nondenaturing zwitterionic detergent for memebrane biochemistry: design and synthesis. Proc Natl Acad Sci USA. 77: 6368-6370.
- Connor RJ, Engler H, Machemer T, Philopena JM, et al. Identifaction of polyamides that enhance adenovirus-mediated gene expression in the urothelium. Gene Ther 2001; 8(1): 41-48.
- Kuball J, Wen SF, Leissner J, Atkins D, Quijano E, et al. Successful adenovirus-mediated wild-type p53 gene transfer in patients with bladder cancer by intravesical vector instillation. J Clin Oncol 2002; 20: 957-965.
- Hu Y, Wu B, Zillmer A, Lu P, Benrashid E et al. Guanine analogues enhance antisense oligonucleotide-induced exon skipping in dystrophin gene in vitro and in vivo. Mol Ther 2010; 18(4): 812-818.
- Sun J, Zeng F, Jian H, Wu S. Conjugation with Betaine: A Facile and Effective Approach to Significant Improvement of Gene Delivery Properties of PEI. Biomacromolecules 2013; 14(3):728-736.
- Inácio S, Mesquita KA, Baptista M, Ramalho-Santos J et al. In vitro surfactant structure-toxicity relationships: implications for surfactant use in sexually transmitted infection prophylaxis and contraception. PLoS One 2011; 6(5):e19850.
Citation: Wang M, Wu B, Drains M, Lu P, Lu Q (2020) Neutral Surfactant Enhanced Exon-skipping of Morpholino Oligonucleotides in vitro and in MDX Mice. J Nanomed Nanotech. 11:553. doi: 10.35248/2157-7439.20.11.553
Copyright: © 2020 Wang M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited