Research Article - (2020) Volume 8, Issue 1
Pyrrolizidine alkaloids (PAs) are phytotoxins produced by plants as a secondary metabolite against herbivores. PAs account for acute hepatotoxic, mutagenic, carcinogenic and teratogenic effects in humans and animals. In vivo PA intoxications of humans and animals are well documented all over the globe. The current study investigated the toxicological chemical make-up of Ageratum conyzoides L, collected from North Western Zones of Tigray region of Ethiopia, where episodic outbreak of hepatic veno-occlusive disease (HVOD) occurred. During the episodic outbreak multiple hundred deaths were reported due to PAs intoxications. PAs contamination occurred through food items such as millet in the farm fields. This study has led to take interventional measures against the causative agents to cease the morbidity and mortality. The identified PAs possesses the 1:2 double bond in the pyrrole ring necessary for in vivo activation and subsequent liver intoxications. The presence of Angeloyl-platynecine, Angeloyl-retronecine, and the macrocyclic, Seneciphylline alkaloids in A. conyzoides L. is not known previously. Isolation of the different PAs were realized by column chromatography (CC) with silica gel, sephadex-LH-20 for size exclusion CC and cationexchanger (CE). PA detection was performed by TLC with Ehrlich reaction method of Mattocks and their identification with gas-chromatography/mass-spectrometry (GC-MS) equipped with standard PAs library. PA containing plants and compounds should be under the watchlist to prevent similar outbreaks worldwide.
Pyrrolizidine alkaloid; Ageratum conyzoides; HVOD; Human intoxication
PAs are phytotoxins to humans and animals biosynthesized exclusively by plants, so far, circa. 600 different PAs are known occurring in about 3% of all flowering plants [1].secondary metabolites as a defense mechanism against herbivores [2]. Incidents of several PAs intoxications were reported in humans and animals [3-5].
PA intoxications in humans and livestock are understood to be sub-chronic and chronic type, liver being their target organ; it requires a certain length of time for the electrophilic pyrrole to build-up to PA induced outbreak usually in a form of hepatic veno-occlusive disease [1].
PAs are frequently found in two forms, their N-oxide (PANO) and as tertiary base PAs. Many PAs, nearly 100 are known to display toxicity such as carcinogenic and mutagenic properties giving rise 10 to 30-fold enlargements of the liver cells and in many cases led to liver damage and death. Chemically, PAs are derivatives of 1-methyl-pyrrolizidine, consisting of two fused fivemembered rings with bridgehead nitrogen atom at position 4 to form a heterocyclic nucleus [3]. When hydroxylated, this structure is referred to as a necine which can either be saturated or contain a double bond in the 1, 2 position. Necine bases may also be N-methylated, forming otonecine. The necine moiety is often esterified to constituents called necic acids, which vary significantly in structure.
According to the reports from the regional health bureau, a total of 1095 cases (302 deaths and 793 under clinical follow-up) were reported in the North West Zone of Tigray. Case fatality rate was 27.6% [4]. The sites where the A. conyzoides L. were collected are communities seriously affected by HVOD episodic outbreak in villages of North-West zone of Tigray region, Ethiopia, that caused significant morbidity and mortality in humans and animals. This investigation was undertaken with the aim of identifying the types of PAs contained in this invasively grown species and correlate with the intoxicants to the liver disease so as to take interventional measures with the possible causative agent to contain the health problem.
Isolation and identification of pas
A. conyzoides L. were collected during the month of June from the HVOD affected areas of the region. The development stage of the plant was in flowering stage. The plant was identified by the Botanist in EPHI and specimen deposited at the institute’s herbarium. The plant material was dried under shade and grounded to fine powder (weight 2 kg) and extracted three times with 80% aqueous methanol (2000 ml) using maceration. The resulting extract was evaporated under vacuo and combined to afford 500 g of oily crude extract which was kept in amber colored bottle with a lid in a refrigerator until used for analysis.
Acid-base enrichment of pyrrolizidine alkaloid
Acid-Base PA enrichment of the crude extract was as follows, 70 g of the crude extract dissolved in aqueous hydrochloric acid (1 M, 600 ml), was shaken and washed with dichloromethane (5 × 300 ml) to remove non-alkaloids, the acidic fraction was made alkaline with concentrated ammonia (pH 9) and extracted with dichloromethane (5 × 500 ml) to afford PA fraction one, this fraction were then dried with sodium sulphate and dried under vacuo. The residual ammoniacal solution was made acidic and stirred with 30 g of zinc dust for 3 hours, followed by centrifugation and decantation, the resulting supernatant was made alkaline with excess ammonia and extracted with dichloromethane (5 × 500 ml) fraction two. Both alkaloid fractions were combined to afford 25 g enriched pyrrolizidine alkaloid fraction which was used for Column Chromatography.
Column chromatography
15 grams of the acid-base enriched pyrrolizidine fraction was adsorbed in silica and subjected to CC carefully packed with 150 g of silica gel using mobile phase, chloroform: methanol (CHCl3-CH3OH) successive gradient system, (100:1-1:1) to provide 20 fractions which was analyzed on the basis of their thin layer chromatography (TLC) and combined. The combined enriched PAs was dissolved in 60 ml of methanol and applied to a clean and equilibrated strong cation exchanger (SCE) and allowed for 50 minutes to facilitate the exchange of alkaloids. The column was eluted with the same solvent and continued with saturated NaCl. The NaCl fraction was extracted with methanol to afford highly enriched PAs fraction as determined by spraying the developed TLC with Ehrlich reagent which was then applied to Sepahedex LH-20 to afford, Ac_Tg_1, Ac_Tg_2, and Ac_Tg_3.
Instrumental condition of GC-MS
Hewlett Packard 5890, Series II, NPD-Detector, Auto-sampler 7673 A. PC-system Chromeleion V.6.20. Fused-Silica-Gel capillary column, Optima-5, DF-60 m, 01.25 μm X ID. He; splitless, 1.5 PSI. Inj./Det.-Temp; 300ºC; Temperature program (180-280ºC; 5ºC/min; Endt: 15 min.). Injection: 2 μl. Hewlett Packard 5890, Series II, Quadrupol MS, auto-sampler 7673. Injection 3 μl. Column CP-Silm 8, 50 m, 0.25 μm × 0.25 mm. MS: Source: 180°C, Interface: 290ºC; Injector: 290ºC; Full-scan mode. Identification was performed with standard PAs library matching.
Not all PAs are toxic to humans and animals only those possessing a 1,2 double-bond in their base moiety (necine) are hepatotoxic, carcinogenic, genotoxic, teratogenic and sometimes pneumotoxic (13), corroborating with our findings that out of the seven identified PAs in A. conyzoides L. samples, six of them contain the 1,2 double-bond of necine base moiety, (Figures 1-3), which is a predisposing factor for the occurrence of hepatic VOD leading to the significant motility and mortality occurred in the affected community as a result of liver failure due to necrosis and liver dysfunctions. Histopathological studies conducted in sixty-one HVOD affected patients revealed the occurrence of the diseases in the studied region [6,7].
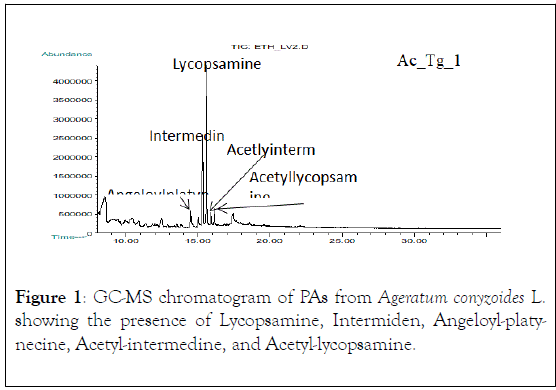
Figure 1: GC-MS chromatogram of PAs from Ageratum conyzoides L. showing the presence of Lycopsamine, Intermiden, Angeloyl-platynecine, Acetyl-intermedine, and Acetyl-lycopsamine.
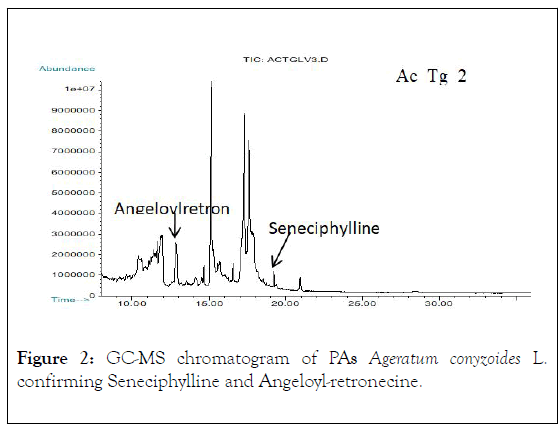
Figure 2: GC-MS chromatogram of PAs Ageratum conyzoides L. confirming Seneciphylline and Angeloyl-retronecine.
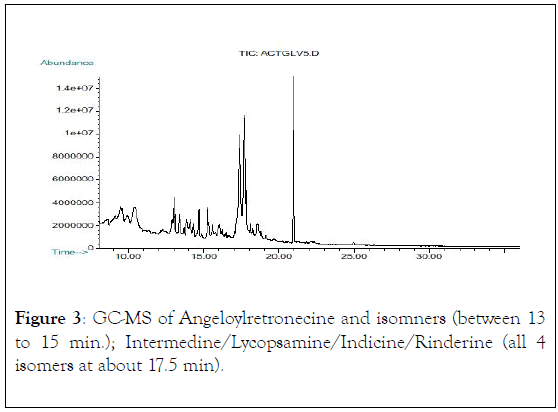
Figure 3: GC-MS of Angeloylretronecine and isomners (between 13 to 15 min.); Intermedine/Lycopsamine/Indicine/Rinderine (all 4 isomers at about 17.5 min).
Human and animal intoxications of PAs are known across the globe, more recently in Israel with Heliotropium europaeum where 24 cows were reported dead as a result of PAs contamination on their fodder hay over a period of two months [5], The repeated HVOD epidemic in Afghanistan due to consumption of bread made from wheat contaminated with PAs containing seeds of Heliotropium popovii, during the outbreak more than 1600 individuals showed HVOD and the mortality rate was 20% from an exposed inhabitants of 35000 in approximately 100 villages and in the second outbreak a total of 44 deaths were reported [3,8]. The case fatality rate in the affected villages in the Norther West Tigray were 27.6% [4].
The following toxic pyrrolizidine alkaloids were identified from the hydro-alcoholic extracts of A. conyzoides L. collected from HVOD affected communities, known PAs such as Lycompsamine, Intermedine, Acetly-Lycopsamine, Acetlyintermedine, and unknown in the plant were Angeloylretronecine, Angeloyl-platynecine, and Seneciphylline (the macrocyclic diester PA) Figure 4.
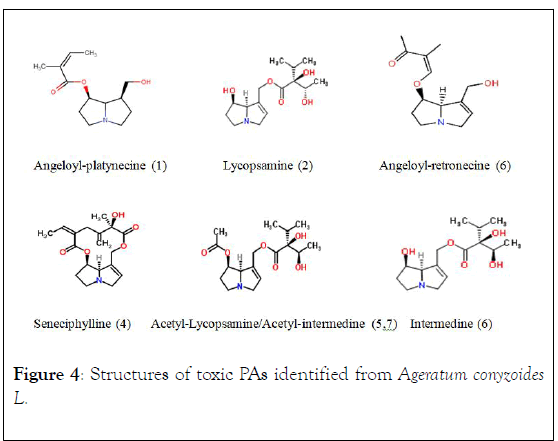
Figure 4: Structures of toxic PAs identified from Ageratum conyzoides L.
The presence of Angeloyl-platynecine, Angeloyl-retronecine, and the macrocyclic, Seneciphylline alkaloids in A. conyzoides L. is not known previously. PAs usually occur in very low concentrations; however, toxin concentrations are significantly higher in invasive weed species than in native species. A. conyzoides is native to tropics and highly invasive, especially under drought conditions, which are alien to its natural environment, this shifting defense mechanism further support the high amount of PAs identified in this study [2].
Previously identified toxic alkaloids from the plant A. conyzoides across various geographic locations include lycopsamine, echinatine, acetyl-echinatine, clivorine (macro-cyclic diester type), dihydro-lycopsamine, and acetyl-lycopsamine, and Noxides and other phenolic compounds are reported in the literature [6,9,10]. It’s pharmacological and biological properties as well as essential oils for insecticide is reviewed in detail. The presence of macrocyclic diester PA from A. conyzoides L. specifically the authors identified the presence of clivorine alkaloid, corroborating with our finding of Seneciphylline [10,11].
The contamination of food grain samples such as millet with A. conyzoides L collected from the affected communities. Quantified concentration of up to 87.1 μg PAs in 182 grams of millet, collected from the affected households. The normal daily consumption amount to approximately 100 g millet/person. This PAs concentrations are higher than the limitation set by The European Food Safety Authority with a benchmark dose of 70 μg/kg body weight per daily intake for the induction of PA intoxication. The normal daily consumption of millets were approximately 100 g millet/person, moreover, the highest number of deaths occurred in families that used contaminated millet for food and traditional alcoholic beverage [8].
Considering the maximum daily intake dose of toxic PAs to 10 μg in herbal preparations and 1 μg for other preparations set by the German health institution/Bundes-gesundheithsamt, the daily consumption of toxic PAs in the affected communities of 87.1 μg exceed the limit more than ten times. The identification of structurally diverse toxic alkaloids in this study from the affected villages corroborate with the high number of deadly outbreaks of hepatic VOD [12].
The scientific community was curious to know the types of PAs contained in the Ethiopian A. conyzoides , after the report of HVOD outbreak in villages. Researchers in the USA tried to attempt to link the observed episodic outbreak of liver damage to PAs by phytochemically investigating A. conyzoides collected from unrelated geographic location in Hawaii, USA. Though this is an appreciable attempt to know the PAs in that specific location, however, is highly unlikely to show a complete chemical similarity in PAs make-up of A. conyzoides collected from HVOD reported villages in the region. This is mainly because of shifting defense mechanism of the weed, where it has invasively grown in the region after years of arid climatic conditions and in such conditions plants are known to produce toxins in high amounts with diverse chemical structures in order to thrive in such environment [3,8].
The toxicity of PAs is mainly due to in vivo activation, they undergo a 3-step metabolic activation in the liver indicating liver is their first site for activation and toxicity as outlined in Figure 5. The first step being attachment of hydroxy-group by hepatic cytochrome P-450 monooxygenase enzyme to produce the biotransformation products (IIa and IIb), and secondly, dehydration of hydroxylated-PAs to didehydro-pyrrolizidine alkaloids products (DHPAIK/III), followed by rearrangement to electron reach reactive aromatic pyrrole which reacts with nucleophilic component of a cell to produce the observed toxification. N-oxide PAs are also converted to the toxic form after oral ingestion either by gut enzymes or liver microsomes to the free bases rendering them to be equally toxic to that of the free necine bases and necic acid [1]. Recent studies, have showed the potential of PAs to penetrate to the nucleus and react with the cell components, resulting a formation of DNA cross-links and DNA-protein cross-links thereby disrupting the normal cell functions and damage to the hepatocyte [5,13].
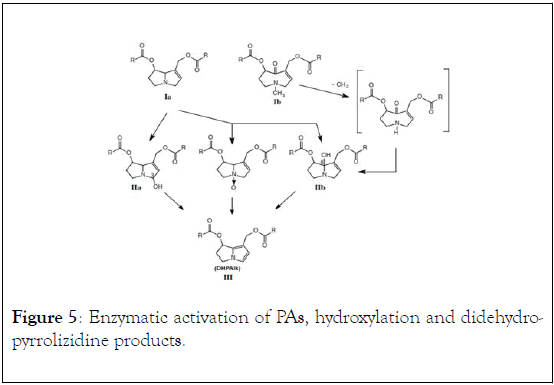
Figure 5: Enzymatic activation of PAs, hydroxylation and didehydropyrrolizidine products.
Finally, our investigation to the causative agents to the fatal episodic outbreak in the form of HVOD and the subsequent deaths in humans and animals were attributed to the contamination of food grains and animal fodders with toxic pyrrolizidine alkaloids contained in the invasively grown weed of A. conyzoides L. in the farm fields of studied area. Consequently, interventional actions of physically removing the weed from the farm fields and animal fodder were carried out to avert further intoxication and thereby terminating the deaths in humans and animals.
PAs contamination of food grains and animal fodder were responsible for the observed episodic outbreak. The present study identified hepatoxic and tumorigenic PAs in A. conyzoides L. from hepatic veno occlusive disease affected communities (HVOD). Accordingly, interventional measures on the toxic weed of A. conyzoides from the grain farms and animal fodder were taken to prevent additional losses of humans and animals in the studied area. The chromatographic and spectroscopic identification of toxic PAs clearly confirms the chemical correlation to the fatal episodic outbreak of HVOD. Therefore, such kinds of toxic molecules should be under the watchlist as hazardous substances by the wider scientific communities across the globe to prevent similar fatal outbreaks proactively.
The author is grateful for financial support of the study supported by Ministry of Finance and Economic Development through Ethiopian public health Institute (EPHI). I would like to extend a warm regard to Dr. Helmut Wiedenfeld of Pharmaceutical Institute, University of Bonn, An der Immenburg 4, D-53121 Bonn, Germany for the GC-MS analysis of PAs.
Citation: Belete Y, Wondu K, Wiedenfeld H, Debella A (2020) Natural Toxins of Ageratum Conyzoides from Hepatic Vein Occlusive Disease Affected Community in Ethiopia. Nat Prod Chem Res. 8:370. DOI: 10.35248/2329-6836.20.8.370
Received: 03-Feb-2020 Published: 24-Feb-2020, DOI: 10.35248/2329-6836.20.8.370
Copyright: © 2020 Belete Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.