Indexed In
- Open J Gate
- Genamics JournalSeek
- CiteFactor
- Cosmos IF
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- Directory of Abstract Indexing for Journals
- OCLC- WorldCat
- Proquest Summons
- Scholarsteer
- ROAD
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2019) Volume 11, Issue 3
Metadicholî A Novel Inverse Agonist of Thyroid Receptor and its Applications in Thyroid Diseases
Raghavan PR*Received: 19-Nov-2018 Published: 09-Dec-2018, DOI: 10.35248/0974-8369.19.11.458
Abstract
An estimated 200 million individuals worldwide have a thyroid disorder. Thyroid diseases affect seven times more women than men. People not diagnosed make up the majority of thyroid patients. There is a need to find novel and safe ways to change the underlying disease processes, rather than merely stop excess thyroid hormone production as in hypothyroidism.
Metadichol® is a nano emulsion of an extract of long-chain alcohols from food that is an inverse agonist of VDR (Vitamin D receptor), AHR (Aryl Hydrocarbon Receptor), and RORC (RAR Related Orphan Receptor C). The work presented here shows that Metadichol® is an inverse agonist of THRA (Thyroid Receptor Alpha) and THRB (Thyroid Receptor Beta). Case studies are presented that show how it can safely treat a multitue of thyroid related diseases. Network and pathway enrichment studies are presented that show how Metadichol® may be involved in action on multiple receptors and exerting its effects through multiple pathways. Metadichol® is the first of a breed of molecules that moves the goal post from the concept of ‘one drug, one target’ toward simultaneously targeting multiple targets, that can potentially lead to successful treatment of many diseases. Given the safety profile of Metadichol®, it would not only mitigate thyroid disease but prevent it and reducing the burden on healthcare budgets worldwide.
Keywords
VDR (Vitamin D receptor); Metadichol; Nano emulsion; Thyroid nuclear receptors; THRA (Thyroid Receptor Alpha); THRB (Thyroid Receptor Beta); Inverse and Protean agonists; Hypothyroidism; Hyperthyroidism; Thyroid antibodies; Hashimoto; Graves’ disease
Introduction
Thyroid disease affects about 12 percent of the US population. While many people with thyroid disease don't even know they have it, an overactive or underactive thyroid can cause a slew of problems, including weight gain or loss, mood changes and infertility. In children, an underactive thyroid can be fatal, which is why they are tested for a deficiency at birth [1,2].
On a global scale, a staggering 200 million people have problems with their thyroid glands, with over 50 percent remaining undiagnosed. It affects women more disproportionately than men. Thyroid hormones are essential for growth, neuronal development, reproduction and regulation of energy metabolism. Hypothyroidism and hyperthyroidism are common conditions with potentially devastating health consequences that affect all populations worldwide.
The thyroid hormone receptors, THRA and THRB are members of the nuclear receptor family [3]. The body relies heavily on the thyroid hormone for digestion, the growth of hair and nails, sex drive, and metabolism are dependent on the right levels of thyroid hormone for proper functioning. The thyroid uptakes iodine from diet and converts it into thyroid hormones.
The two main types of thyroid hormones are called L-triiodothyronine (T3) and L-thyroxine (T4). These hormones regulate control how cells, use oxygen and energy. The synthesis and secretion of thyroid hormones are controlled by feedback loops that involve the hypothalamus, pituitary, and thyroid glands.
The hypothalamus secretes Thyrotropin-Releasing Hormone (TRH), that signals the pituitary, that releases Thyroid-Stimulating Hormone (TSH), signaling the thyroid gland to synthesize thyroid hormones. High thyroid hormone levels will inhibit TRH production, decrease TSH secretion from the pituitary, and decrease thyroid hormone synthesis [4].
Thyroid diseases and treatment
Today four drugs are currently being used for treating thyroid diseases 3,5 diiodothyropropionic acid, amiodarone, L-triiodothyronine, Levothyroxine.
Hypothyroidism is a disorder with multiple causes in which the thyroid fails to secrete an adequate amount of thyroid hormone, this is the most common thyroid disorder usually caused by primary thyroid gland failure and may result from diminished stimulation of the thyroid gland by TSH. Hypothyroidism can be treated with thyroid hormone replacement drugs. Levothyroxine is a stereoisomer of thyroxine (T4) which is degraded much slowly and can be administered once daily in patients with hypothyroidism [5]. Hyperthyroidism results in excess synthesis and secretion of thyroid hormones by the thyroid gland. The synthetic form of the T3 hormone, known as liothyronine is sometimes added to levothyroxine for T4/T3 combination treatment (Table 1).
Table 1: Typical thyroid hormone levels in thyroid disease.
| TSH | T4 | T3 | |
|---|---|---|---|
| Hypothyroidism | High | low | low |
| Hyperthyroidism | low | high | high |
Hashimoto's is an autoimmune disease, meaning that the immune system inappropriately attacks your thyroid gland. Thyroid-attacking antibodies produced are Thyroid Peroxidase antibodies (TPO) and Thyroglobulin Antibodies (TgAb). Hashimoto disease leads to conditions that cause inflammation and gradual destruction of thyroid gland over time leading to hypothyroidism.
Graves' disease is an autoimmune disorder that causes hyperthyroidism, or overactive thyroid. This is caused by overstimulation of the thyroid. Graves’ disease leads to goiter and in some cases thyroid nodules. This leads to cancer of the thyroids that is most often found in nodules in the thyroid gland. Hyperthyroidism caused by Graves' disease may be treated with the thioamide drugs propylthiouracil, carbimazole or methimazole, and rarely with Lugol's solution. Additionally, hyperthyroidism and thyroid tumors may be treated with radioactive iodine.
Thyroid cancer almost always involves removal of the thyroid gland surgically or need Radioactive Iodine treatment (RAI). Patients after surgery are hypothyroid and require lifelong thyroid hormone replacement treatment. In some cases, thyroid diseases and conditions can have no symptoms at all, such as thyroid cancer or certain types of thyroiditis.
Thyroid diagnosis
Diagnosis of a thyroid condition is usually by blood tests or imaging tests. Blood testing: Levels of (TSH) test, free thyroxine (Free T4) and free triiodothyronine (Free T3) and TPO, TgAB testing and for reverse T3 levels. Imaging tests include CT, MRI and ultrasound scans were carried out to further evaluate the size, shape, and function of the thyroid gland.
There is today a paucity of drugs for treating thyroid diseases and a need for clinically safe molecule. Thyroid antagonists in general have dual THRA and THRB activities. Development of selective THRA and THRB small molecule antagonists has not been considered clinically relevant. A specific small molecule thyroid antagonist is, therefore, a desirable goal of the pharmaceutical industry to treat hyperthyroidism. An increase in circulating thyroid agonists will lead to hyperthyroidism. Whereas a loss in thyroid receptor gene signaling leads to hypothyroidism. Compounds exhibiting thyroid antagonism can cause serious side effects. Drugs amiodarone have thyroid antagonist properties and can induce hypothyroidism in some people.
Thyroid and role of cytokines
A thyroid disease is an inflammatory state characterized by elevated cytokines. Modulating cytokine responses hold considerable promise in the treatment of thyroid diseases [6]. Xinyang et al. showed that constitutively activated NF-κB pathway also closely links Hashimoto's thyroiditis with increased incidence of thyroid cancers and inhibition of NF-κB, showed alone or with other signaling pathway inhibitors may be of significant therapeutic benefits against aggressive thyroid cancers [7]. Diez et al. reported that high plasma concentrations of TNF-alpha and sTNFR-I are seen in patients with hypothyroidism or hyperthyroidism [8]. Fukazawa et al. showed Intercellular Adhesion Molecule 1 (ICAM1) in the sera of patients with Grave’s Disease [9]. This feature may be an important factor in the progression of cancer of the thyroid gland. Similar work was reported by Zhang et al. that ICAM1 expression is upregulated in PTC (Papillary Thyroid Cancer) and in HT (Hashimoto’s disease) [10]. Buitrago et al. showed that ICAM1 was upregulated in PTC at the levels of both gene and protein expression. Moreover, this upregulated expression correlated with aggressive tumor features [11].
Kemp et al. [12] confirmed expression of CCL2 (MCP-1), levels were associated with serum levels of antithyroid peroxidase (anti- TPO Ab) and antithyroglobulin antibodies (anti-TG Ab). This was confirmed by Wahl et al. [13]. Kokkotou et al. [14] showed that serum CCL2 is increased in women with chronic autoimmune thyroiditis, with family history of hypothyroidism, suggesting a pathogenetic role for CCL2 in this condition as confirmed by another study by Didushko et al. [15].
Thyroid and vitamin D
Research to date suggest the association of vitamin D deficiency with higher incidence of autoimmune thyroiditis [16,17]. More severe deficiency is often accompanied by thyroid hypofunction [18]. The levels of 25(OH)D3 seem to be an independent factor influencing the presence of TPOAb positivity [19,20].
It has been vitamin D below 12.5 ng/mL is a risk factor for development of thyroid diseases. Whether supplemented with high doses of vitamin D is preventive or could have therapeutic effect is still being debate [21]. It has been shown by molecular modelling studies that 1,25-D has a very high affinity [22] for the alpha Thyroid Nuclear Receptor (THRA). Since Metadichol® is an inverse agonist of VDR (Vitamin D Receptor), a thyroid nuclear receptor assay was carried out. It showed that Metadichol is an interestingly an inverse agonist of THRA and THRB. Metadichol is also inhibitor of TNF alpha, MCP1 (CCL2) and ICAM1 [23,24] have a role in thyroid diseases and presented here are case studies that confirm Metadichol® and its potential use in mitigating Thyroid diseases.
Methods
Experimental nuclear receptor assay
Nuclear receptor (NR) assays: The assays were performed by Indigo Biosciences Inc, PA, USA using their proprietary assay methods.
Plasmids: This study utilized a hybrid receptor comprising the N-terminal Gal4 DNA binding domain fused to the ligand binding domain of the specific human nuclear receptor (THRA and THRB). The reporter vectors used in these studies comprise the firefly luciferase gene functionally linked to either an upstream NR Response Element (NRE) or the Gal4 activation sequence (UAS).
Compound Handling: Test compound was delivered as solution dissolved in water. Stock of test compounds was stored at room temperature.
Set up of NR Assays: The NR Assays were performed as depicted in Figures 1 and 2.
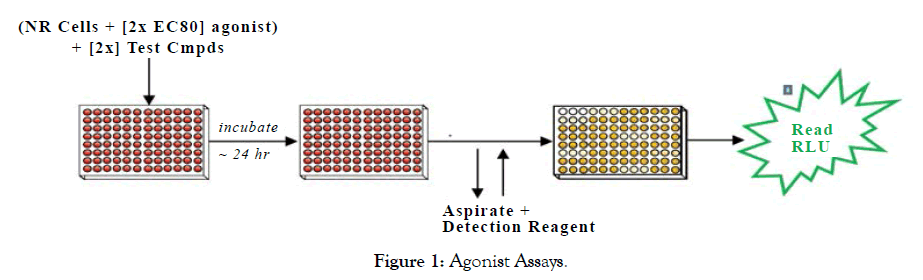
Figure 1: Agonist Assays.
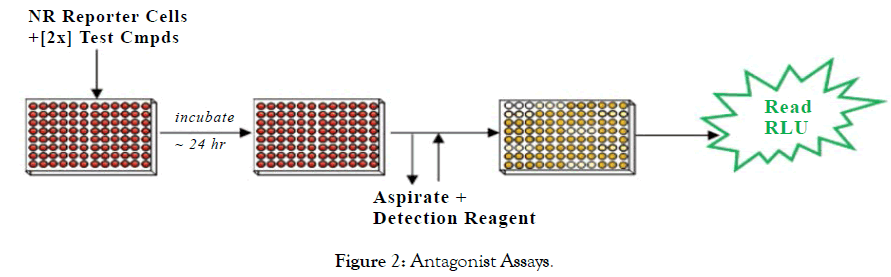
Figure 2: Antagonist Assays.
Step 1: A suspension of Reporter Cells was prepared in Cell Recovery Medium (CRM; containing 10% charcoal stripped FBS). For antagonist assays, reporter cells were supplemented with a 2x-EC80 concentration of the appropriate reference agonist. For agonist and antagonist assays, 100 mL of the reporter cell suspension was dispensed into the wells of white, cell culture treated, 96-well assay plates. For agonist and antagonist assays, 2x-concentration treatment media were prepared.
Step 2: Immediately prior to assay setup, test compounds were diluted using Compound Screening Medium (CSM; containing 10% charcoal stripped FBS) to generate '2 x-concentrations' treatment media. 100 mL of each treatment medium was dispensed into triplicate assay wells pre-dispensed with Reporter Cells. Assay plates were incubated at 37°C for 24 hours.
Step 3: Following the 24 hour incubation period, treatment media were discarded and 100 all/well of Luciferase Detection Reagent was added. RLUs were quantified from each assay well to determine NR activity.
Assay validation
Reference compounds were utilized to confirm the performance of the specific lot of NR Reporter Cells treated with the Sponsor's test compounds. Reference compound and test compound assays were performed at the same time and, hence, were exposed to the same assay reagents and environmental conditions. Refer to individual data sets for the identities of specific reference agonist and antagonist and their respective treatment concentration ranges. Reference groups always include a 'Vehicle' control to determine background activity in the assay and to calculate fold activation or percent-inhibition.
Data reduction
Microsoft Excel was used to manage and archive assay data, as well as to calculate average RLU values +/- Standard Deviation (SD), Fold-activation, Percent activation, Percent Coefficients of Variation (%CV), and Z' values. Graphical data methods (Table 2).
Table 2: Graphical data methods.
| • Percent Coefficient of Variation (%CV) is: | 100*(SD/Ave. RLU) |
| • Fold-Activation in Agonist assays is: | Ave RLU Test Cmpd/Ave RLU Vehicle |
| • Percent-Activation of Ref Max is calculated by normalizing test cmpd RLU values to the maximum RLU value of the reference agonist (=100%), as follows: | 100*(Ave. RLU Test Cmpd/Ave RLU Reference maximum) |
| • Z' for Reference Agonists is: | 1-[(3*[SD Vehicle + SD Ref Cmpd]) / (RLU Ref Cmpd - RLU Vehicle)] |
| • Fold-Inhibition in Antagonist is: | [Ave RLU EC80 agoinst / Ave RLU Test Cmpd] |
| • Percent-Inhibition in Antagonist: | The theoretical minimum inhibition (0% inhibition) derives from EC80 agonist treatment only, no treatment cmpd. % Inhibition is calculated as: 100*( 1-[Ave RLU Test Cmpd / Ave RLU EC80 agoinst) |
Dose Response Curve (DRC) analyses of the reference compounds and test compounds were performed via non-linear curve-fitting of Fold-activation vs. compound for agonist assays. Percent inhibition vs. compound for antagonist and inverse agonist assays. Graph Pad Prism software was used for calculations (Tables 3-6) (Figures 3-8).
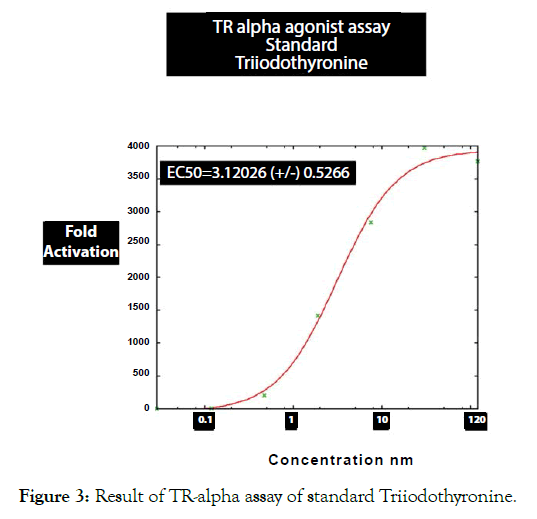
Figure 3: Result of TR-alpha assay of standard Triiodothyronine.
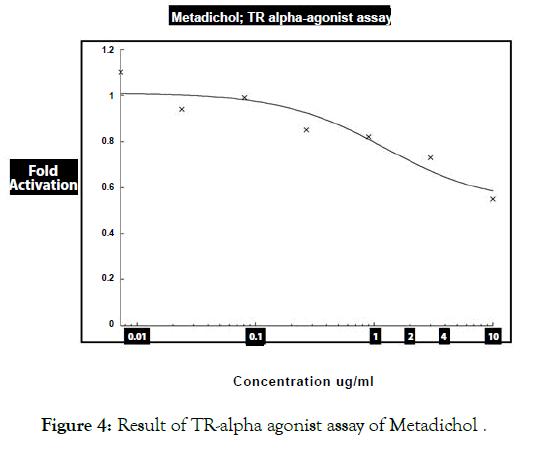
Figure 4: Result of TR-alpha agonist assay of Metadichol .
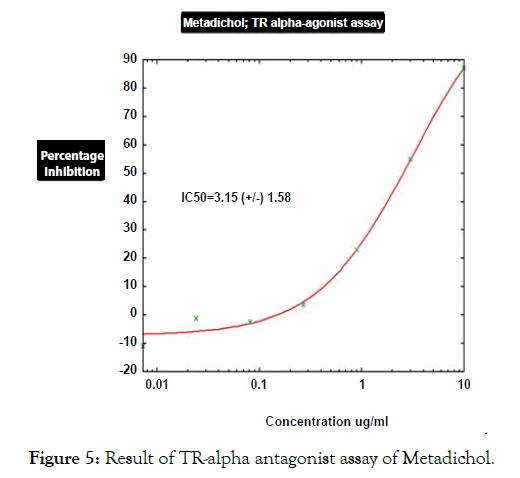
Figure 5: Result of TR-alpha antagonist assay of Metadichol.
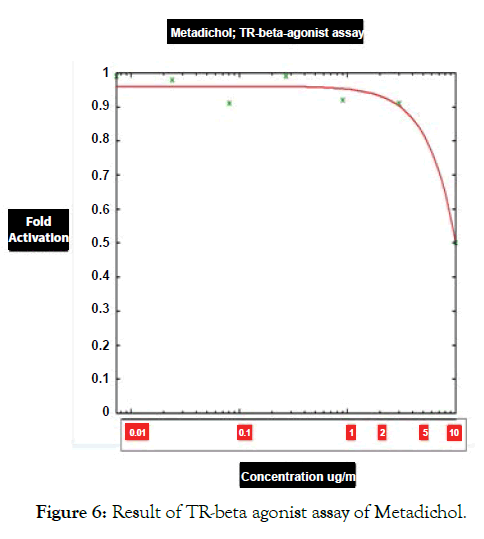
Figure 6: Result of TR-beta agonist assay of Metadichol.
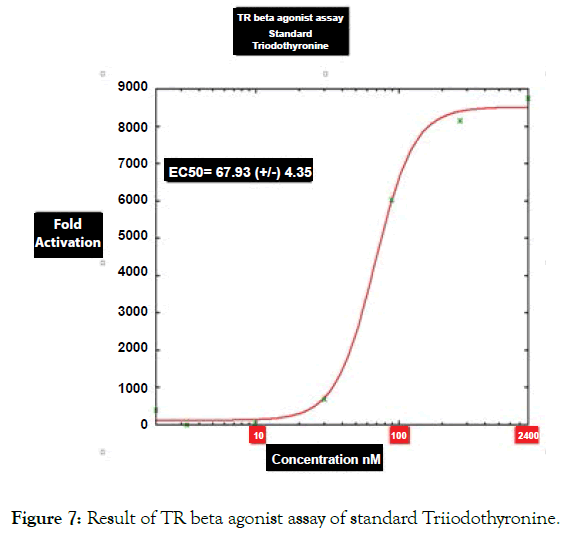
Figure 7: Result of TR beta agonist assay of standard Triiodothyronine.
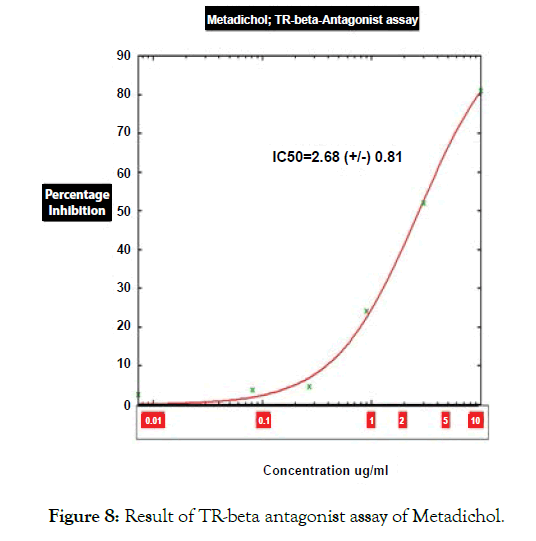
Figure 8: Result of TR-beta antagonist assay of Metadichol.
Table 3: Results of TR alpha agonist assays of Metadichol and reference agonist Triiodothyronine.
| Compound | Conc. | Human TRα Agonist Assays, Host cell line: HEK | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Luc1 | Luc2 | Luc3 | AVG | SD | Fold-Activation | %Activation of Ref Max | % CV | |||
| Water | 0.20% | 386 | 254 | 318 | 319 | 66 | 1 | 0.025 | 21 | |
| Metadichol (mg/mL) | 0.0073 | 342 | 326 | 428 | 365 | 55 | 1.1 | 0.029 | 15 | |
| 0.024 | 328 | 270 | 302 | 300 | 29 | 0.94 | 0.024 | 9.7 | ||
| 0.081 | 288 | 306 | 354 | 316 | 34 | 0.99 | 0.025 | 11 | ||
| 0.27 | 254 | 280 | 282 | 272 | 16 | 0.85 | 0.021 | 5.7 | ||
| 0.9 | 288 | 266 | 228 | 261 | 30 | 0.82 | 0.021 | 12 | ||
| 3 | 286 | 206 | 204 | 232 | 47 | 0.73 | 0.018 | 20 | ||
| 10 | 224 | 144 | 156 | 175 | 43 | 0.55 | 0.014 | 25 | ||
| Reference Agonist: Triidothyronine (nM) | 0.029 | 526 | 454 | 394 | 458 | 66 | 1.4 | 0.036 | 14 | Z' |
| 0.12 | 3,335 | 2,734 | 2,186 | 2,752 | 574 | 8.6 | 0.22 | 21 | ||
| 0.47 | 72,967 | 64,891 | 61,845 | 66,568 | 5,748 | 208 | 5.3 | 8.6 | 0.74 | |
| 1.9 | 6,00,780 | 3,91,861 | 3,67,490 | 4,53,377 | 1,28,235 | 1,420 | 36 | 28 | 0.15 | |
| 7.5 | 10,58,970 | 8,51,942 | 8,07,433 | 9,06,115 | 1,34,234 | 2,837 | 71 | 15 | 0.56 | |
| 30 | 14,56,630 | 12,32,700 | 11,13,220 | 12,67,517 | 1,74,332 | 3,969 | 100 | 14 | 0.59 | |
| 120 | 16,47,590 | 12,56,070 | 11,46,010 | 12,01,040 | 77,824 | 3,761 | 95 | 6.5 | 0.81 | |
Table 4: TR alpha antagonist assays of Metadichol and reference agonist triiodothyronine.
| Compound | Conc. | Human TRβ Antagonist Assays HEK | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Luc1 | Luc2 | Luc3 | AVG | SD | Fold-Inhibition | %Inhibition | % CV | ||
| Water | 0.20% | 10,00,450 | 10,17,700 | 10,43,760 | 10,20,637 | 21,804 | 1 | 0 | 2.1 |
| Metadichol (mg/mL) | 0.0073 | 9,50,092 | 9,99,788 | 10,31,020 | 9,93,633 | 40,814 | 1 | 2.6 | 4.1 |
| 0.024 | 9,37,815 | 10,93,660 | 11,11,230 | 10,47,568 | 95,454 | 0.97 | -2.6 | 9.1 | |
| 0.081 | 9,08,237 | 9,82,324 | 10,56,140 | 9,82,234 | 73,952 | 1 | 3.8 | 7.5 | |
| 0.27 | 9,37,650 | 9,81,457 | 10,01,420 | 9,73,509 | 32,619 | 1 | 4.6 | 3.4 | |
| 0.9 | 7,85,643 | 7,45,300 | 7,81,945 | 7,70,963 | 22,301 | 1.3 | 24 | 2.9 | |
| 3 | 5,14,370 | 4,67,836 | 4,85,441 | 4,89,216 | 23,496 | 2.1 | 52 | 4.8 | |
| 10 | 1,98,260 | 1,89,334 | 2,01,774 | 1,96,456 | 6,413 | 5.2 | 81 | 3.3 | |
Table 5: TR beta antagonist assays of Metadichol and reference agonist triiodothyronine.
| Compound | Conc. | Human TRα Antagonist Assays, Host cell line: HEK | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Luc1 | Luc2 | Luc3 | AVG | SD | Fold-Inhibition | %Inhibition | % CV | ||
| Water | 0.20% | 8,03,254 | 8,07,166 | 8,29,297 | 8,13,239 | 14,044 | 1 | 0 | 1.7 |
| Metadichol (ug/mL) | 0.0073 | 8,71,672 | 8,70,226 | 9,68,181 | 9,03,360 | 56,142 | 0.9 | -11 | 6.2 |
| 0.024 | 8,61,060 | 7,68,219 | 8,42,466 | 8,23,915 | 49,122 | 0.99 | -1.3 | 6 | |
| 0.081 | 7,74,125 | 8,28,213 | 8,96,931 | 8,33,090 | 61,548 | 0.98 | -2.4 | 7.4 | |
| 0.27 | 7,35,981 | 8,31,026 | 7,86,600 | 7,84,536 | 47,556 | 1 | 3.5 | 6.1 | |
| 0.9 | 6,04,043 | 6,26,890 | 6,56,405 | 6,29,113 | 26,252 | 1.3 | 23 | 4.2 | |
| 3 | 3,53,284 | 3,32,801 | 4,17,058 | 3,67,714 | 43,943 | 2.2 | 55 | 12 | |
| 10 | 89,689 | 1,14,198 | 1,08,014 | 1,03,967 | 12,746 | 7.8 | 87 | 12 | |
Table 6: TR beta agonist assays of Metadichol and reference agonist triiodothyronine.
| Compound | Conc. | Human TRβ Agonist Assays, Host cell line: HEK | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Luc1 | Luc2 | Luc3 | AVG | SD | Fold-Activation | % Activation of Ref Max | % CV | |||
| Water | 118 | 132 | 154 | 135 | 18 | 1 | 0.012 | 13 | ||
| Metadichol | 0.0073 | 124 | 120 | 156 | 133 | 20 | 0.99 | 0.012 | 15 | |
| (ug/mL) | ||||||||||
| 0.024 | 144 | 108 | 142 | 131 | 20 | 0.98 | 0.012 | 15 | ||
| 0.081 | 110 | 134 | 122 | 122 | 12 | 0.91 | 0.011 | 9.8 | ||
| 0.27 | 142 | 136 | 120 | 133 | 11 | 0 Compound.99 | 0.012 | 8.6 | ||
| 0.9 | 104 | 120 | 148 | 124 | 22 | 0.92 | 0.011 | 18 | ||
| 3 | 106 | 80 | 182 | 123 | 53 | 0.91 | 0.011 | 43 | ||
| 10 | 60 | 68 | 74 | 67 | 7 | 0.5 | 0.006 | 10 | ||
| Reference Agonist: Triiodothyronine (nM) | 3.3 | 176 | 172 | 174 | 174 | 2 | 1.3 | 0.015 | 1.1 | Z' |
| 9.9 | 2,140 | 1,942 | 1,652 | 1,912 | 245 | 14 | 0.17 | 13 | 0.55 | |
| 30 | 97,190 | 95,283 | 81,040 | 91,171 | 8,826 | 677 | 8.1 | 9.7 | 0.71 | |
| 89 | 7,92,651 | 8,52,420 | 7,91,517 | 8,12,196 | 34,840 | 6031 | 72 | 4.3 | 0.87 | |
| 267 | 10,89,770 | 10,91,930 | 11,09,530 | 10,97,077 | 10,839 | 8,147 | 97 | 1 | 0.97 | |
| 800 | 12,62,850 | 11,56,450 | 11,12,770 | 11,77,357 | 77,193 | 8,743 | 104 | 6.6 | 0.8 | |
| 2400 | 10,71,220 | 10,86,870 | 12,26,570 | 11,28,220 | 85,532 | 8,378 | 100 | 7.6 | 0.77 | |
Table 7: Enriched pathways of THRA and THRB generated by EVINET.
| Gene set | Number of links | Enriched pathways | FDR (False Discovery Rate) | Reactome pathway |
|---|---|---|---|---|
| THRA, THRB | 321 | Regulation of Thyroid hormone | 8.60E-292 | R-HSA-350864 |
| THRA, THRB | 321 | Transcriptional regulation of white adipocyte differentiation | 2.20E-112 | R-HSA-381340 |
| THRA, THRB | 321 | Thyroxine biosynthesis | 1.40E-82 | R-HSA-209968 |
| THRA, THRB | 321 | Xenobiotics | 1.60E-61 | R-HSA-211981 |
| THRA, THRB | 321 | CYP2E1-reactions | 3.50E-48 | R-HSA-211999 |
Results of Experimental Assays
Results of case studies
Patient No 1: Male-38
38 year old male with episodes of syncope associated with seizure activity. It all began with a routine Hepatitis B injection and soon after he began getting seizures. Neurological tests were normal. Was initially reated with anticonvulsants Dilantin and Keppra but the seizure episodes have continued? In addition he suffered from double vision, auras and episodes of dizziness. Most episodes occurred during night while sleeping. He usually collapses, and is completely unconscious for a period of time (15-30 minutes) during which time he may or may not have any movements. He returns to full rational consciousness after the end of these seizures. Needed to be hospitalized after such episodes he used to feel tired and rests for 1-2 days before he feels normal. He was finally diagnosed with Hashimoto encephalopathy with elevated levels of Thyroid antibodies. Treated with Metadichol @ dosage of 10 mg per day in addition to other medications and began to show improvements.
Though seizures continued the frequency lessened and with Metadichol uses so far only mild seizure for 30 seconds and patient returned to normal after 10 minutes as opposed to 1-2 days before and without need to be hospitalized. This improvement was reflected in his biomarkers levels. Patient reports normal blood pressure. Frequent seizures have decreased and since starting with Metadichol. Patient has stopped use of all medications. Patient reports he has very energetic and feels less stressed mentally about his condition (Figures 9-14).
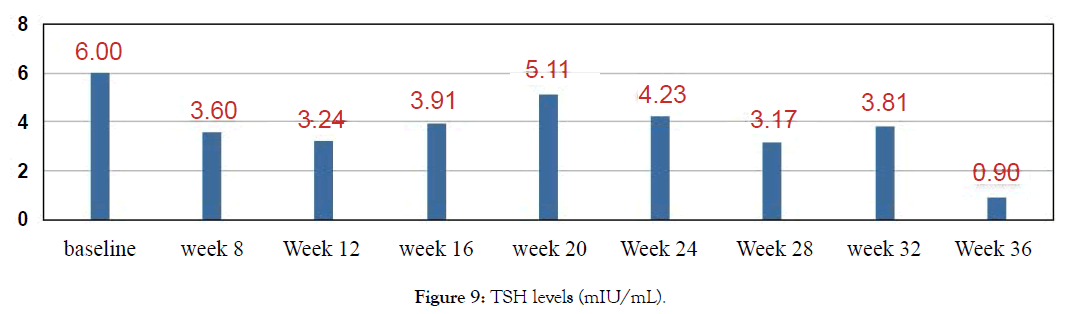
Figure 9: TSH levels (mIU/mL).
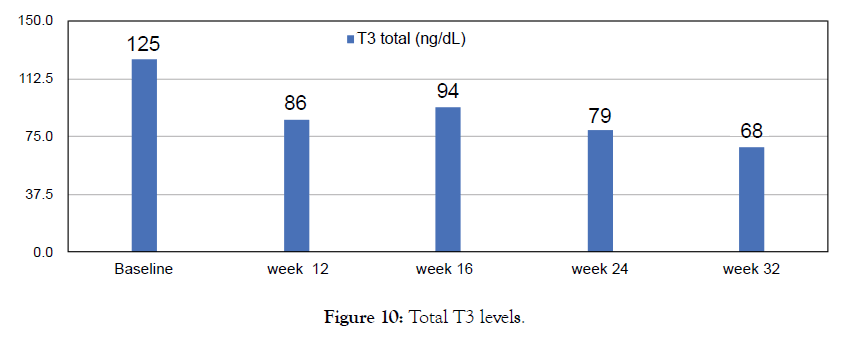
Figure 10: Total T3 levels.
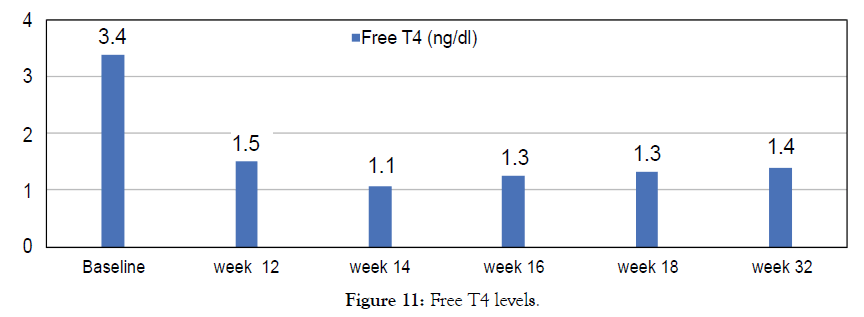
Figure 11: Free T4 levels.
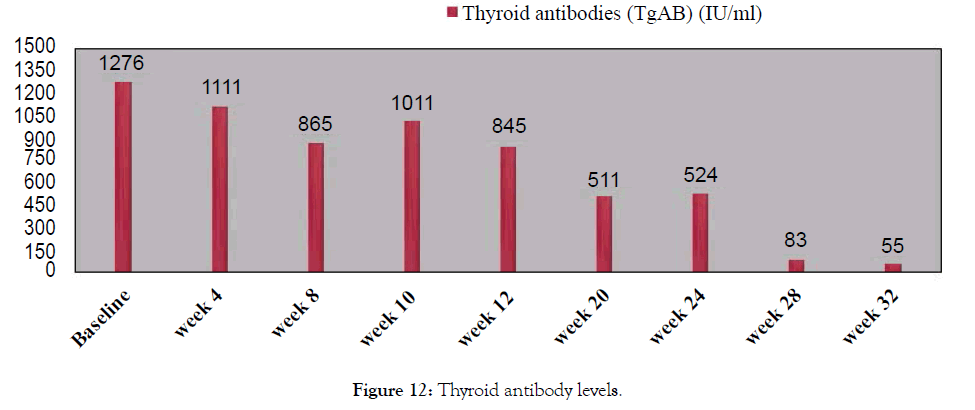
Figure 12: Thyroid antibody levels.
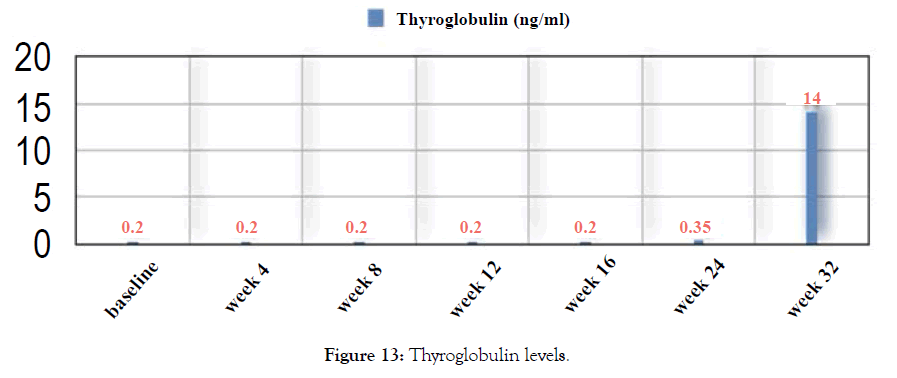
Figure 13: Thyroglobulin levels.
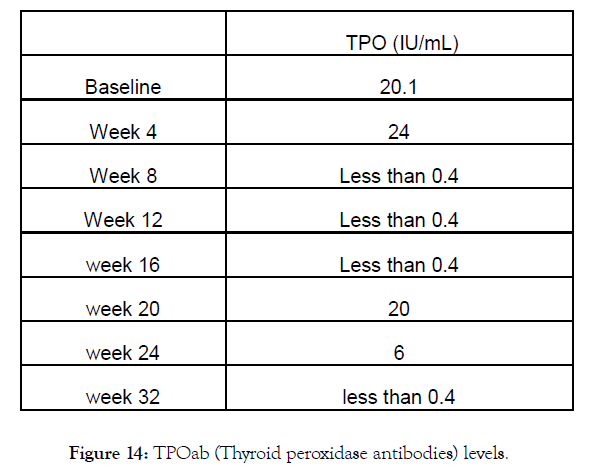
Figure 14: TPOab (Thyroid peroxidase antibodies) levels.
Patient No 2: Male-83
Male-83, lung cancer patient. Metadichol @ 10 mg per day. Patient was on chemotherapy and not on any thyroid medications. TSH levels returned to normal range (Figure 15).
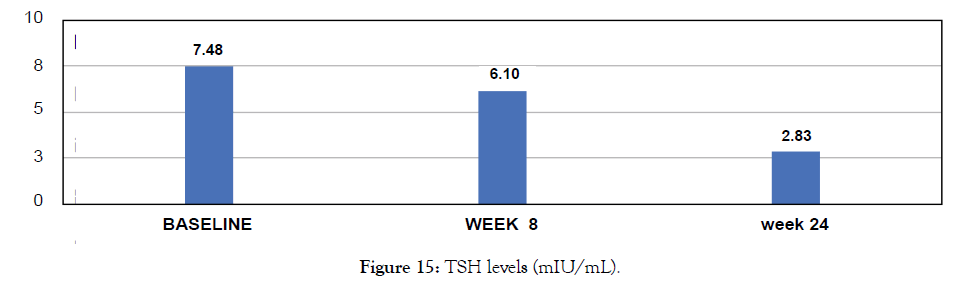
Figure 15: TSH levels (mIU/mL).
Patient No 3: Female-74
74 years old female with hypothyroid and co morbidities of cardiovascular diseases, anemic and weak with low energy levels not on any Thyroid medication. Patient reports improved energy levels after use of Metadichol @ 5 mg per day (Figure 16).

Figure 16: TSH levels (mIU/mL).
Patient No 4: Female-51
Female 51 diagnosed with hypothyroidism was not on any Thyroid medication. Metadichol® treatment at 5 mg per day. Patient normalized over 20 weeks (Figure 17).
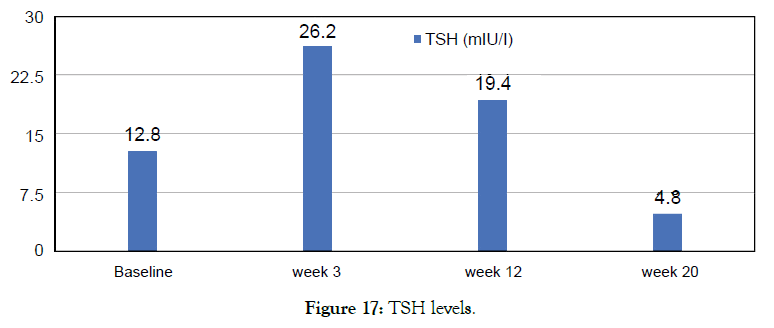
Figure 17: TSH levels.
Patient No 5: Female-27
Female 27 diagnosed with hypothyroidism. Was not on any thyroid medication. Metadichol treatment @ 5 mg per day improved TSH levels. Patient still on Metadichol. Patient reports improved energy levels (Figures 18 and 19).
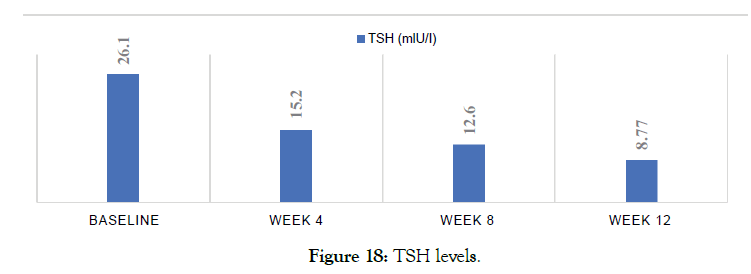
Figure 18: TSH levels.
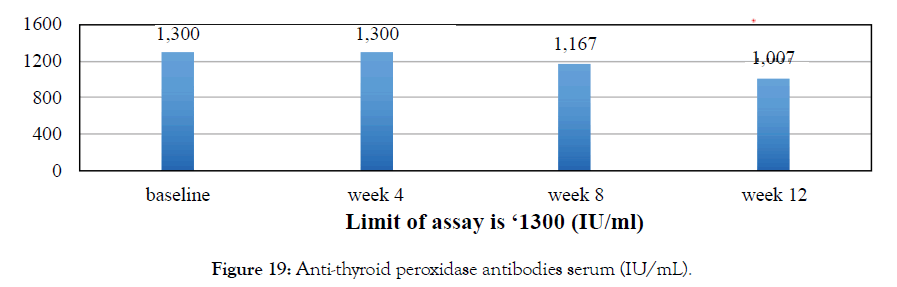
Figure 19: Anti-thyroid peroxidase antibodies serum (IU/mL).
Patient No 6: Female-27
Female 27 diagnosed with hyperthyroidism. Her doctor prescribed Carimazol but she opted to use Metadichol. After 4 months all her Thyroid biomarkers returned normal. She stopped Metadichol use at 10 month and a year later at month 22 her biomarkers are still in the normal range (Figures 20 and 21).
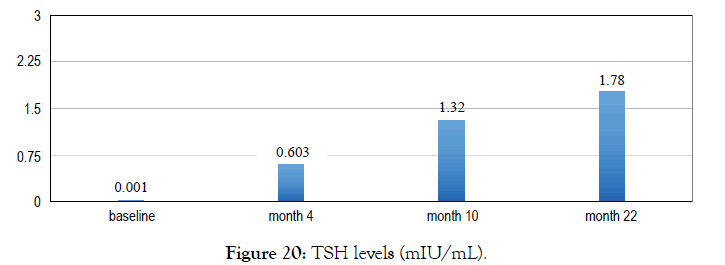
Figure 20: TSH levels (mIU/mL).
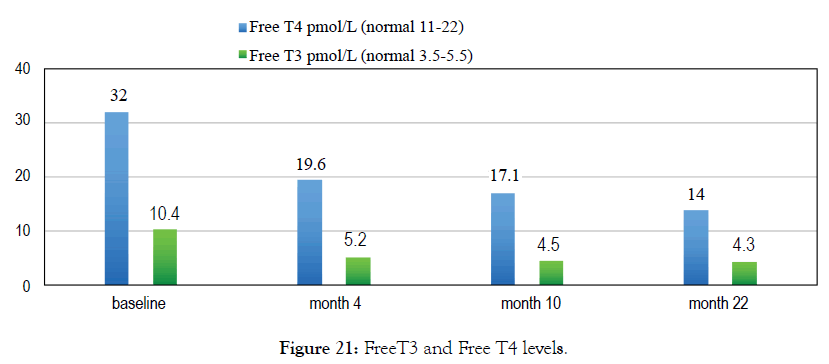
Figure 21: FreeT3 and Free T4 levels.
Discussion
The cases presented all showed improvement in all thyroid biomarkers panel. Metadichol binds to thyroid nuclear receptors both THRA and THRB but as an inverse agonist. There are no known inverse agonists of thyroid nuclear receptors known in literature today. For inverse agonism to exist there must be constitutive activity present i.e. receptors are actively signaling in the absence of an agonist [25]. Related to inverse agonism is what is referred to as protean agonism [26] such ligands display both agonist and inverse agonist properties. Efficacy of a ligand is depending on the level of receptor constitutive activity of the receptor in the assay system used. Therefore, a partial agonist in one system can behave as an antagonist or an inverse agonist in others. For protean agonism to exist there needs to be constitutive receptor activity i.e. inverse agonism that is seen with Metadichol®. The results show that it is effective against both hyperthyroidism and hypothyroidism and normalizing T3, T4 and levels of antiobodies. It is likely that Metadichol® is behaving as a protean agonist. Metadichol is also an inverse agonist of other nuclear receptors VDR, AHR (Aryl hydrocarbon receptor) and RORC (RAR related Orphan receptor C) [24,27,28].
With the experimental data of binding to the thyroid receptors use a web-based program like EviNet [29] to see the network enrichment analysis of the genes of interest. It allows applicability of genes found in global network and to connect genes via one various molecular mechanism. Using these just 2 genes THRA and THRB led to over 100 significant pathways and the top 5 are shown in Table 7. From this analysis the enriched Reactome pathways [30] from THRA and THRB are linked to 321 genes in the network. Figure 22 shows the network of genes involved with regulation of thyroid hormone pathway with a FDR (False Detection Rate) value of 8.6 E-292.

Figure 22: Pathway R-HSA-350864. Regulation of thyroid hormone activity generated by Evinet.
DIO 1: It catalyzes the activation, as well as the inactivation of thyroid hormone. Responsible for the deiodination of T4 (3,5,3',5'-tetraiodothyronine) into T3 (3,5,3'-triiodothyronine) and of T3 into T2 (3,3’-diiodothyronine) which is inactive.
DIO 2: Catalyzes the conversion T4 to bioactive T3. It has an essential role in providing the brain with appropriate levels of T3 during the critical period of development.
DIO 3: Responsible for the deiodination of T4 into RT3 (3,3',5'-triiodothyronine) and of T3 into T2 (3,3'-diiodothyronine). RT3 and T2 are inactive metabolites.
The network clearly shows how genes DIO 1, 2 and 3 play a role in maintaining appropriate levels of T3 and T4 in regulating the thyroid hormone pathway. It is clear that binding to both THRA and THRB is important in enhancing the thyroid pathway. Metadichol® binding to thyroid nuclear receptors leads to control of TSH and also T3 and T4 levels.
The second pathway that is enriched is transcriptional regulation of white adipocyte differentiation. The adipose tissue is targeted by thyroid hormones as this tissue is where energy stored and acts as a regulator of energy balance, and modulating signals to maintain metabolic control. Thyroid hormones regulate the gene markers of differentiation of adipocytes, lipogenesis, lipolysis, and thermogenesis in the adipose tissue [31].
Research work on use if selective agonist of THRB in lipid related disease though the results have been disappointing [32].
Metadichol also binds to VDR, AHR and ROR gamma as inverse agonists. Given the cross talk that occurs between various nuclear receptors a connected network of nuclear receptors [33] can be generated using a software like Innate DB [34-36] a database of genes, proteins, experimentally-verified interactions and signaling pathways involved in the innate immune response of humans. This is shown in Figure 23. The numbers in the figure are known nuclear receptors. What we see is binding to multiple receptors leads to activating other nuclear receptors in this case 37 out of 48 known in humans. Binding and or activation of these nuclear receptors leads to gene transcription and leads to many pathways and thus many targets. Metadichol® has already been shown to impact many diseases through a multidue of pathways [37].
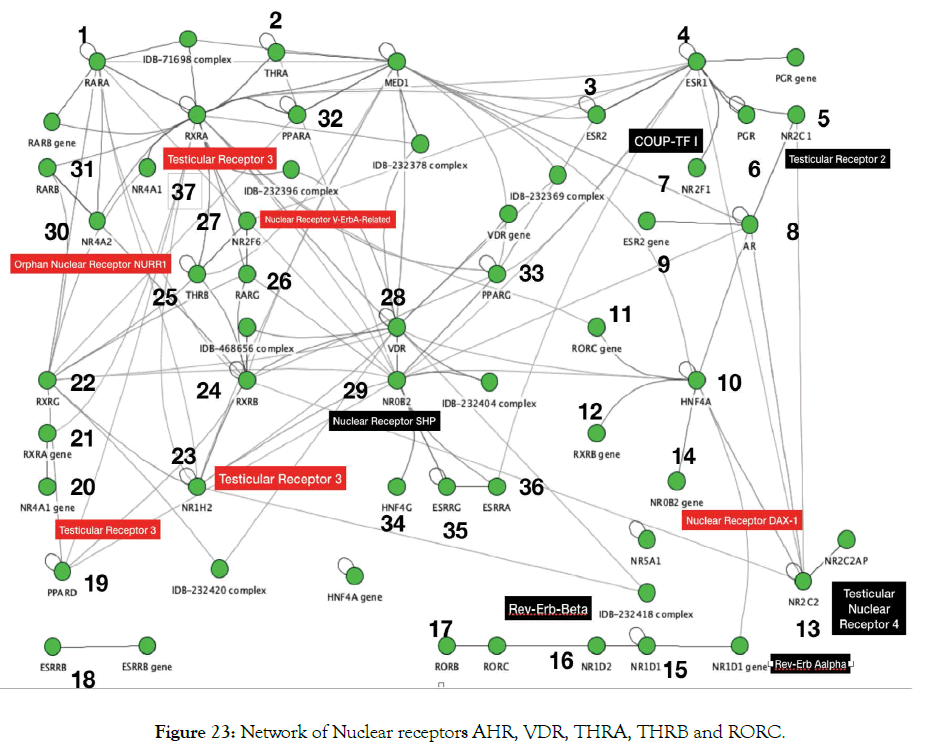
Figure 23: Network of Nuclear receptors AHR, VDR, THRA, THRB and RORC.
A macro level interaction network of TNF alpha, ICAM1 and the nuclear receptors VDR THRA, THRB is shown in Figure 24. This was generated using pathway commons [38]. VDR controls the expression of THRA that interacts with THRB [39]. A more detailed interaction is shown in Figure 25 generated by using InnateDB software.
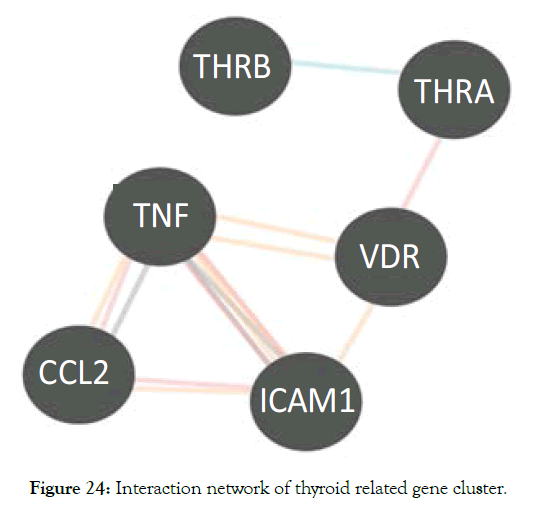
Figure 24: Interaction network of thyroid related gene cluster.
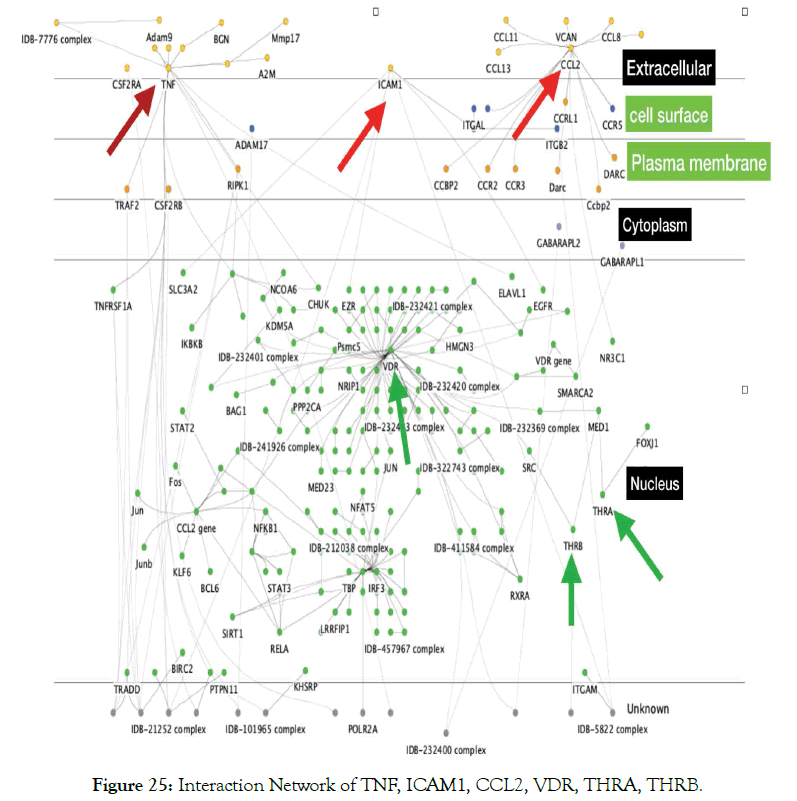
Figure 25: Interaction Network of TNF, ICAM1, CCL2, VDR, THRA, THRB.
Further analysis was carried out using a program like ToppCluster [40] a multiple gene list feature enrichment analyzer for the dissection of biological system. This cluster of six genes (TNF, ICAM1, CCL2, VDR, THRA, THRB) target all the thyroid related diseases as shown in Figure 26. Drugs are more effective if they are modulating multiple genes [41]. All diseases need complex therapeutic approaches. In this respect, as shown above Metadichol ® hits multiple genes belonging to a network of interacting targets and thus has enhanced and efficacy and an enormous advantage when compared to a single-target drug or a combination of multiple drugs [42,43]. Compared to drugs uses in treating diseases Metadichol is a safe and effective nutraceutical to mitigate Thyroid related diseases.
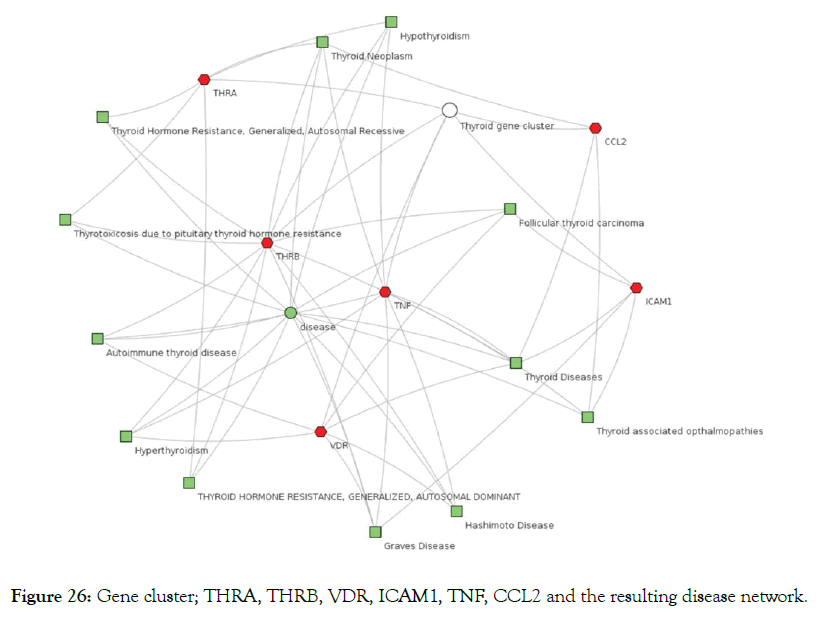
Figure 26: Gene cluster; THRA, THRB, VDR, ICAM1, TNF, CCL2 and the resulting disease network.
Conclusion
Diseases generally are not the result of a single malfunctioning gene or a single malfunctioning pathway. There are many genes that affect the same disease and need to tackle though multiple pathways. The concept of one gene, one disease and one drug are no longer a viable approach. Thus, there is a need to target nuclear receptors which is the operating system of our body. Binding to vitamin D receptor for example is known to affect over 2900 genes and the same is the case with other nuclear receptors. The reason that we have failed to address many diseases adequately in the past was the lack of information regarding how genes are related to each other. Today a vast amount of information is at our finger tips. The key to every biological problem must finally be sought in the cell’s nucleus containing the 48 nuclear receptors which leads to expression of thousands of genes and pathways all interlinked and a road map to a better understanding of diseases and pathways and clues to our understanding our human biological process.
REFERENCES
- Taylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14(5):301-316.
- Vanderpump MPJ. The epidemiology of thyroid disease. British Med Bull. 2011;99:39-51.
- Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81(3):1097-1142.
- Jackson IMD. Thyrotropin-releasing hormone. N Engl J Med. 1982;306:145-155.
- Germain DLS, Hernandez A, Schneider MJ, Galton VA. Insights into the role of deiodinases from studies of genetically modified animals. Thyroid. 2005;15(8):905-916.
- Ganesh BB, Bhattacharya P, Gopisetty A, Prabhakar BS. Role of cytokines in the pathogenesis and suppression of thyroid autoimmunity. J Interferon Cytokine Res. 2011;31(10):721-731.
- Li X, Abdel-Mageed AB, Mondal D, Kandil E. The nuclear factor kappa-b signaling pathway as a therapeutic target against thyroid cancers. Thyroid. 2012;23(2):209-218.
- Díez JJ, Hernanz A, Medina S, Bayon C, Iglesias P. Serum concentrations of tumor necrosis factor-alpha (TNF-alpha) and soluble TNF-alpha receptor p55 in patients with hypothyroidism and hyperthyroidism before and after normalization of thyroid function. Clin Endocrinol (Oxf). 2002;57:515-521.
- Fukazawa H, Yoshida K, Kaise N, Kiso Y, Sayama N, Mori K, et al. Intercellular adhesion molecule-1 (ICAM-1) in the sera of patients with graves' disease: correlation with disease activity and treatment status. Thyroid. 2009;5(5):373-377.
- Zhang K, Ge SJ, Lin XY, Lv BB, Cao ZX, Li JM, et al. Intercellular adhesion molecule 1 is a sensitive and diagnostically useful immunohistochemical marker of papillary thyroid cancer (PTC) and of PTC-like nuclear alterations in Hashimoto's thyroiditis. Oncol Lett. 2016;11(3):1722-1730.
- Buitrago D, Keutgen XM, Crowley M, Filicori F, Aldailami H, Hoda R, et al. Intercellular adhesion molecule-1 cytokine in thyroid carcinoma coexisting with Hashimoto’s thyroiditis (ICAM-1) is up regulated in aggressive papillary thyroid carcinoma. Ann Surg Oncol. 2012;19(3):973-980.
- Kemp EH, Metcalfe RA, Smith KA, Woodroofe MN, Watson P, Weetman AP. Detection and localization of chemokine gene expression in autoimmune thyroid disease. Clin Endocrinol (Oxf). 2003;59(2):207-213.
- Fröhlich E, Wahl R. Thyroid Autoimmunity: Role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521.
- Kokkotou E, Marafelia P, Mantzos EI, Tritos NA. Serum monocyte chemoattractant protein-1 is increased in chronic autoimmune thyroiditis. Metabolism. 2002;51(11):1489-1493.
- Didushko OM. Abnormal production of monocyte chemoattractant protein in patients with primary hypothyroidism. Galicain Med J. 2015;22(3).
- Bizzaro G, Shoenfeld Y. Vitamin D and autoimmune thyroid diseases: facts and unresolved questions. Immunol Res. 2015;61:46-52.
- Kivity S, Agmon-Levin N, Zisappl M, Shapira Y, Nagy EV, Dankó K, et al. Vitamin D and autoimmune thyroid diseases. Cell Mol Immunol. 2011;8(3):243-247.
- Tamer G, Arik S, Tamer I, Coksert D. Relative vitamin D insufficiency in Hashimoto's thyroiditis. Thyroid. 2011;21(8):891-896.
- Bozkurt NC, Karbek B, Ucan B, Sahin M, Cakal E, Ozbek M, et al. The association between severity of vitamin D deficiency and Hashimoto's thyroiditis. Endocr Pract. 2013;19(3):479-484.
- Shin DY, Kim KJ, Kim D, Hwang S, Lee EJ. Low serum vitamin D is associated with anti-thyroid peroxidase in autoimmune thyroiditis. Yonsei Med J. 2014;55:476-478.
- Muscogiuri G, Tirabassi G, Bizzaro G, Orio F, Paschou SA, Vryonidou A, et al. Vitamin D and thyroid disease: to D or not to D? Eur J Clin Nutr. 2015;69(3):291-296.
- Marshall TG. Vitamin d metabolites affect GCR and thyroid nuclear receptors. Nuclear receptors bed to bed side. 2006.
- Raghavan PR. US patent 8,722,093. 2014.
- Raghavan PR. US patent 9,006,292. 2015.
- Kenakin T. Principles: receptor theory in pharmacology. Trends Pharmacol Sci. 2004;25:186-192.
- De Min A, Matera C, Bock A, Holze J, Kloeckner J, Muth M, et al. A new molecular mechanism to engineer protean agonism at a G protein-coupled receptor. Mol Pharmacol. 2017;91(4):348-356.
- Raghavan PR. Metadichol, a novel ROR gamma inverse agonist and its applications in psoriasis. J Clin Exp Dermatol Res. 2017;8:433.
- Raghavan PR. Metadichol ®. A novel inverse agonist of aryl hydrocarbon receptor (AHR) and NRF2 inhibitor. J Cancer Sci Ther. 2017;9:661-668.
- Jeggari A, Alekseenko Z, Petrov I, Dias JM, Ericson J, Alexeyenko A. EviNet: a web platform for network enrichment analysis with flexible definition of gene sets. Nucleic Acids Res. 2018;46:163-170.
- Fabregat A, Sidiropoulos K, Garapati P, Gillespie M, Hausmann K, Haw R, et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2018;4:649-655.
- Obregon MJ. Thyroid hormone and adipocyte differentiation. Thyroid. 2008;18:185-195.
- Jakobsson T, Vedin LL, Parini P. Potential role of thyroid receptor b agonists in the treatment of hyperlipidemia. Drugs. 2017;77:1613-1621.
- Germain P, Altucci L, Bourguet W, Rochette-Egly C, Gronemeyer H. Nuclear receptor superfamily: principles of signaling. Pure Appl Chem. 2003;75:1619-1664.
- Lynn DJ, Winsor GL, Chan C, Richard N, Laird MR, Barsky A, et al. Innate DB, facilitating systems-level analyses of the mammalian innate immune response. Mol Syst Biol. 2008;4:218.
- Breuer K, Foroushani AK, Laird MR, Chen C, Sribnaia A, Lo R, et al. InnateDB: systems biology of innate immunity and beyond - recent updates and continuing curation. Nucl Acids Res. 2013;41:1228-1233.
- Lynn DJ, Chan C, Naseer M, Yau M, Lo R, Sribnaia A, et al. Curating the innate immunity interactome. BMC Syst Biol. 2010;4:117.
- Raghavan PR. A multi gene targeting approach to treating liver diseases with metadichol®. J Cytokine Biol. 2018;3:126.
- Cerami EG, Gross BE, Demir E, Rodchenkov I, Babur O, Anwar N, et al. Pathway commons, a web resource for biological pathway data. Nucleic Acids Res. 2011;39:685-690.
- Garcia-Villalba P, Jimenez-Lara AM, Aranda A. Vitamin D interferes with transactivation of the growth hormone gene by thyroid hormone and retinoic acid. Mol Cell Biol. 1996;16:318-327.
- Kaimal V, Bardes EE, Tabar SC, Jegga AG, Aronow BJ. ToppCluster: a multiple gene list feature analyzer for comparative enrichment clustering and network-based dissection of biological systems. Nucleic Acids Res. 2010;38:96-102.
- Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, Burrell A, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 2010;20:1352-1360.
- Khan S, Lingrel JB. Thematic minireview series on nuclear receptors in biology and diseases. J Biol Chem. 2011;285:38741-38742.
- Burris T, McCabe E. Nuclear receptors and genetic disease 1st (eds.) Academic press. 2000.
Citation: Raghavan PR (2019) Metadichol® A Novel Inverse Agonist of Thyroid Receptor and its Applications in Thyroid Diseases. Biol Med (Aligarh) 11:458.
Copyright: © 2019 Raghavan PR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


