PMC/PubMed Indexed Articles
Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Market Analysis - (2020) Volume 0, Issue 0
Market trajectory in Drug and Vaccine Research
Kai Kauppinen*Received: 02-Nov-2020 Published: 24-Nov-2020
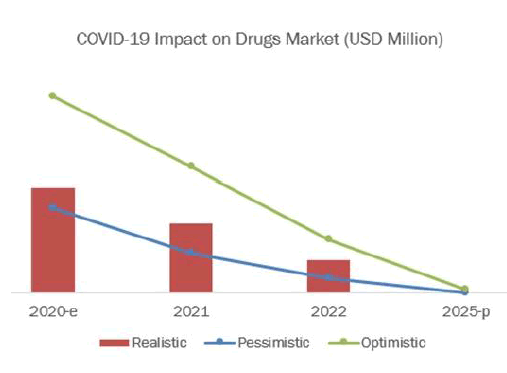
The overall marketplace for vaccines is predicted to grow from USD 36.45 billion in 2018 to USD 50.42 billion by 2023, at a CAGR of 6.7% from 2018 to 2023. Market growth is attributed to the high prevalence of infectious diseases, growing support for vaccine R&D, investments into vaccine development, and a rising specialise in immunization. The base year considered for the study is 2017, and therefore the forecast has been provided for the amount between 2018 and 2023.
Restraints
High cost of vaccine development
Vaccine development is a capital-intensive procedure. The development process (from in vitro research to marketing) takes 10–15 years and an investment of USD 800 million– USD 1 billion. Moreover, because the success rate of vaccine development is extremely low, manufacturers find it challenging to get initial investments and manage operational costs.
Furthermore, the storage and cost of vaccines is above the other pharmaceutical product, because it requires specialized equipment and monitoring devices. The lack of proper storage and distribution facilities can deteriorate vaccine quality. Therefore, this high cost of vaccine development and their storage restrains the market growth.
Opportunities
Use of adjuvants in vaccines
Combined adjuvants are used for increasing the efficacy of vaccines. Companies are researching new adjuvant combinations and novel Immunomodulatory molecules to enhance antigen-specific protection from diseases, such as cancer, hepatitis, HIV, and others. For instance, an ISCOM technology-based combined adjuvant is currently in clinical trials and used for studies on infectious diseases. For instance, in April 2016, the NIAID awarded six grants totaling USD 3.1 million to researchers studying adjuvant vaccine combinations. The increasing significance of such combined adjuvants offers growth opportunities for market players in the coming years.
The following are the major objectives of the study.
1. To define, describe, and forecast the worldwide market on the idea of technology, type, disease indication, route of administration, and patient type
2. To describe and forecast the market, in terms useful, by region–Asia, Europe, North America, and remainder of the planet (RoW) alongside their respective countries
3. To strategically analyze micro markets with reference to individual growth trends, prospects, and contributions to the general market
4. To analyze strategic approaches like product launches, acquisitions, contracts, agreements, and partnerships within the market
The entire procedure includes the study of the annual and financial reports of the highest market players and extensive interviews with industry experts like CEOs, VPs, directors, and marketing executives for key insights (both qualitative and quantitative) pertaining to the market. The figure below shows the breakdown of the primaries on the idea of the corporate type, designation, and region considered during the research study.
Target Audience
Manufacturers and suppliers of vaccines
Research and development companies
Medical research laboratories
Academic medical centers and universities
Research and consulting firms
Venture capital firms
Vaccines Market Report Scope
By Technology
• Conjugate Vaccines
• Inactivated and Subunit Vaccines
• Live Attenuated Vaccines
• Toxoid Vaccines
• Recombinant Vaccines
By Type
• Monovalent Vaccines
• Multivalent Vaccines
By Disease Indication
• Pneumococcal Disease
• Influenza
• DTP
• Hepatitis
• Human Papilloma Virus (HPV)
• Rotavirus
• Meningococcal Disease
• MMR (measles, mumps, and rubella)
• Varicella
• Polio
• Herpes Zoster
• Dengue
• Other Disease Indications
-
International Conference on Robotics, AI & ML


