Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Euro Pub
- Google Scholar
- Quality Open Access Market
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2021) Volume 7, Issue 4
Low Dose Cannabidiol for the Treatment of Mild to Moderate Musculoskeletal Pain
Matthew K Caloura*, Debra Kimless, Susan Lewis and Stephen GoldnerReceived: 19-Jul-2021 Published: 09-Aug-2021, DOI: 10.35248/2684-1320.21.7.156
Abstract
Sixteen healthy, cannabis and cannabidiol (CBD) naïve patients with mild to moderate musculoskeletal pain took a single dose of 5 mg CBD sublingual tablet. Patients were using other-the-counter (OTC) medications to treat various manifestations of musculoskeletal pain, including neck, back, muscle aches, and arthritis. The average self-reported pain scale score (NPRS), measured on a scale of 0-10, was 5.13 prior to taking the CBD tablet. After tablet administration, the patients self-reported their pain scale score over a 2-hour period. All patients reported pain reduction within 20 minutes after taking the tablet and continued over the 2-hour period where the average reported pain was reduced by 20-fold (NPRS 5.13 to 0.25) and was statistically significant (p<0.001). No patients reported any adverse effects and characterized the experience as positive. The results of this study suggest that low dose CBD is an effective option for the treatment of mild to moderate musculoskeletal pain.
Keywords
Cannabidiol; Musculoskeletal pain; CBD; Pain
Introduction
According to the CDC, in 2016, approximately 4 million people experience mild to moderate pain musculoskeletal pain. Chronic pain, even mild to moderate, can adversely impact quality of life, the ability to move or exercise and was linked to numerous physical and mental conditions that contributes to high health care costs and lost productivity [1]. Adverse effects with the use of over-the-counter pain relievers are well described. Acetaminophen has been shown to cause liver damage and may contribute to dementia, stroke, and cardiovascular disease [2]. Chronic use of prescription or over-the-counter NSAIDs were found to lead to dyspepsia, gastritis, ulcers, renal failure, hypertension, stroke, arrhythmia, and heart attack [3]. Recent studies have shown that CBD has a low rate of adverse effects and is relatively safe as compared with the adverse effects of acetaminophen and non-steroidal anti-inflammatory drugs [4]. There are few studies examining cannabis and cannabinoids for the treatment of mild to moderate musculoskeletal pain. A study in a rodent model with muscular pain using synthetic cannabinoids showed that interacting with cannabinoid receptors either systemically or locally, may reduce pain signaling that contributes to musculoskeletal pain [5]. Although not addressing musculoskeletal pain, animal studies also show that cannabinoids reduce pain associated with diabetic neuropathy [6]. In a diabetic rat model study, acute treatment with CBD exerted a significant anti-allodynic effect, which was not associated with locomotor impairment [7]. The mechanism of action is postulated to involve non-endocannabinoid 5HT1A receptors. Clinical studies with cannabis and cannabinoids, and specifically CBD, have reported that cannabinoids or cannabis could be an effective treatment of chronic musculoskeletal pain, as well as to reduce or replace opioids for pain management [8,9] in humans.
Case Study
This case report represents 16 patients who were all treated with a single dose, 5 mg CBD sublingual tablet. Every patient reported mild to moderate musculoskeletal pain for which they were using OTC medications. The OTCs were either NSAIDs or acetaminophen for pain management; however, OTC medications are not without risks [2,3]. CBD, however, may pose an absorption challenge when not inhaled because, as with all cannabinoids, it is a fat-soluble molecule. The tablets administered to the 16 subjects are in a water-soluble, sublingual administration form. The purpose of the sublingual method of administration is to avoid the liver's first-pass effect, improving therapeutic outcomes and efficacy through enhanced bioavailability. Although this case report does not attempt to examine mechanisms of action, its purpose is to examine whether CBD at low dosages could be a potential tool to treat mild to moderate musculoskeletal pain.
Sixteen (16) patients provided written informed consent to take a single dose, 5 mg sublingual CBD tablet. The patients had to have a NPRS score of 3 or greater in order to participate. Each patient must also have had pain for greater than or equal to 10 days of each month for 3 months prior to study entry for which the pain requires treatment. The patients had an age range between 22 and 89 (7 male; 9 female) and represented various manifestations of musculoskeletal pain, including neck pain, back pain, muscle aches, arthritis pain, and dysmenorrhea. The patients did not have any additional significant medical conditions nor were they taking any additional pain medications. Patients documented their NPRS score prior to taking study drug (T-0), and then every 20 minutes post dose for an hour and then at the two-hour timepoint, (T-20, T-40, T-60, and T-120). The NPRS measurements were statistically assessed following completion of the trial using mean calculations, as well as Student’s paired T-test. This study was approved by Allendale Institutional Review Board (AIRB) and was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). Written informed consent was obtained from all subjects prior to inclusion into the trial.
Results
Over the 2-hour timeframe, patients experienced reduction in their pain after a single oral dose of the 5 mg CBD tablet. At T-20, subjects reported significant reduction in pain from moderate to tolerable (2.0) and reported an average NPRS score of 0.25 (no pain) at T-120 (Figure 1).
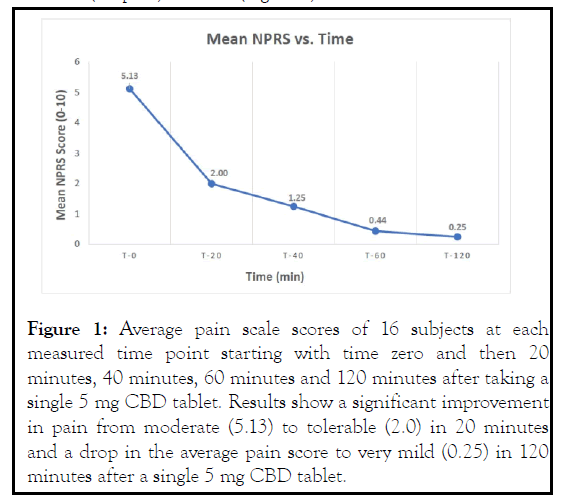
Figure 1: Average pain scale scores of 16 subjects at each measured time point starting with time zero and then 20 minutes, 40 minutes, 60 minutes and 120 minutes after taking a single 5 mg CBD tablet. Results show a significant improvement in pain from moderate (5.13) to tolerable (2.0) in 20 minutes and a drop in the average pain score to very mild (0.25) in 120 minutes after a single 5 mg CBD tablet.
All subjects showed significant reductions in pain at each time point following the baseline NPRS assessment. On average, the reductions demonstrated that the 5 mg dose was effective in management of pain over the duration of the single dose study (p<0.001). Assessment of the individual subject scores also showed that each subject experienced a significant reduction of pain within 120 minutes (Figure 2).
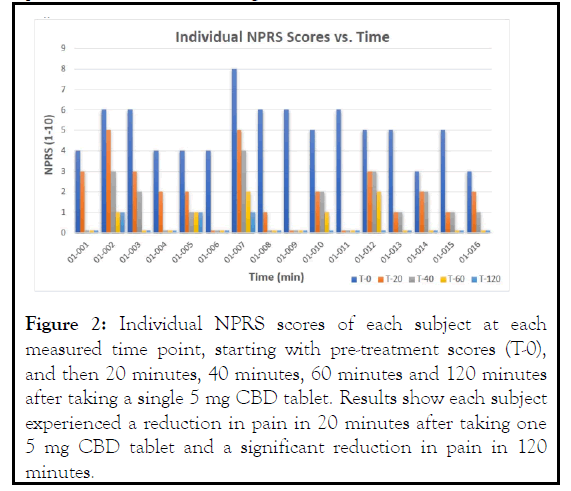
Figure 2: Individual NPRS scores of each subject at each measured time point, starting with pre-treatment scores (T-0), and then 20 minutes, 40 minutes, 60 minutes and 120 minutes after taking a single 5 mg CBD tablet. Results show each subject experienced a reduction in pain in 20 minutes after taking one 5 mg CBD tablet and a significant reduction in pain in 120 minutes.
The 5 mg CBD tablet was well tolerated by the 16 patients with no reported adverse effects. The patients experienced an overall reduction of pain from an NPRS score of 5 to 0.25, or a 20- fold reduction, and reported an overall positive experience. Most patients reported pain relief lasting 4-6 hours, and two subjects reported 24 hours of pain relief.
Discussion
The results of this limited study were unexpected in that all 16 patients responded to the pain-relieving effects of CBD and was statistically significant. We understand that the ability to apply these results to a broader population is limited by the small sample size of this trial (n=16). Further research utilizing a larger sample is needed to verify the reliability of potential data generated from this cannabis-related pain study. However, the results may provide some insight regarding how low dose CBD may be used to treat mild to moderate pain and may be considered a substitute for other traditional pain medications.
The authors will use this case report as a starting point to design additional studies to understand if the results were spurious or if CBD can be considered a therapeutic option for the treatment of mild to moderate pain. Future studies will take into account the duration of the trial in the study design. This study collected results over a two-hour time frame in a single 24-hour period, which may have impacted the reliability of this data. Disease specific pain indications will also be explored; dose escalation studies will be evaluated to confirm dosing as it relates to pain from specific diseases and measure efficacy and safety over time. The effects of cannabinoid treatment on secondary endpoints, such as anxiety reduction and sleep quality, will also be explored. Finally, the methodological choices were constrained by a lack of blinding or randomization. This study utilized an open-label, single arm design. Future studies will consider a randomized, double-blind study in order minimize bias.
Conclusion
CBD is an interesting therapeutic option, especially for the treatment of pain. We understand the limitations of this case report where only 16 subjects used a single low dose CBD tablet for the treatment of mild to moderate musculoskeletal pain. However, the results were statistically significant. This case report may suggest that using low dose CBD could be a safe and effective initial therapy for patients with mild to moderate musculoskeletal pain.
Acknowledgements
The authors would like to thank all investigators, study teams, and patients for participating in this study.
Conflicts Of Interest
The authors are associated with Pure Green Pharmaceuticals, Inc, which developed and manufactured the Pure Green® CBD 5 mg tablets.
REFERENCES
- Dahlhamer J, Lucas J, Zelaya, C, Nahin R, Mackey S, DeBar L, et al. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults — United States, 2016. MMWR Morbidity and Mortality Weekly Report. 2018; 67(36):1001–1006.
- Roberts E, Delgado Nunes V, Buckner S, Latchem S, Constanti M, Miller P, et al. Paracetamol: not as safe as we thought? A systematic literature review of observational studies. Annals of the Rheumatic Diseases. 2015; 75(3):552–559.
- Marcum ZA, Hanlon JT. Recognizing the Risks of Chronic Nonsteroidal Anti-Inflammatory Drug Use in Older Adults. Ann Longterm Care. 2010; 18(9):24-27.
- World Health Organization. CANNABIDIOL (CBD) Critical Review Report. In Expert Committee on Drug Dependence Fortieth Meeting, Geneva.
- Sánchez Robles EM, Bagües Arias A, Martín Fontelles MI. Cannabinoids and muscular pain. Effectiveness of the local administration in rat. European Journal of Pain. 2012; 16(8):1116–1127.
- Doğrul A, Gül H, Yıldız O, Bilgin F, Güzeldemir ME. Cannabinoids blocks tactile allodynia in diabetic mice without attenuation of its antinociceptive effect. Neuroscience Letters. 2004; 368(1):82–86.
- Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. British Journal of Pharmacology. 2011; 163(7):1344–1364.
- Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews. 2018;3(3):CD012182.
- Hill KP, Palastro MD. Medical cannabis for the treatment of chronic pain and other disorders: misconceptions and facts. Polish Archives of Internal Medicine. 2017; 127(11):785-789.
Citation: Kimless D, Caloura MK, Lewis S, Goldner S (2021). Low Dose Cannabidiol for the Treatment of Mild to Moderate Musculoskeletal Pain. J Pain Manage Med. 7:156.
Copyright: © 2021 Kimless D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.