Review Article - (2020) Volume 8, Issue 3
Chemical contamination of water from a wide range of toxic compounds, especially heavy metals, is a critical environmental problem due to their potential human toxicity. Therefore, development of technologies that allow the removal of toxic pollutants from wastewater is a need. Among all the common heavy metals treatment processes, biosorption is one of the more popular and efficient processes. The important number of publications concerning adsorption and remediation of metal ions from aqueous solution by natural and modified polysaccharides shows an increasing interest in biopolymer uses for environment preservation. The present review describes recent developments on biosorption processes using agricultural by-products, mainly mono- and polysaccharides extracted from natural resources. General considerations on metal ions complexation mechanism and interactions between metals and saccharides are first described. Then, a review on metal ions biosorption processes and recent development of new polysaccharide-based materials is carried out and shows their high adsorption capacities and their attractive character. Finally, advantages and possible limitations of using such biosorbents for metal ions adsorption process are discussed.
Polysaccharides; Monosaccharides; Metal ions; biosorption; Complexation; Adsorption; Adsorbents; Wastewater treatment
Water pollution due to toxic metals remains an important environmental problem. Heavy metal ions are used in various industries due to their technological importance: metal processing, electroplating, electronics and a wide range of chemical processing industries. Before being released in the environment, the treatment of contaminated wastewaters is of a great importance since heavy metals are not biodegradable and accumulates in the living species with permanent toxic and carcinogenic effects. For example, chromium (VI) is found to be toxic to bacteria, plants, animals and human [1]. Moreover, faced with more and more stringent regulations, metal ions removal from aqueous effluents also becomes a major source of concern and a priority for most industrial sectors. So the legislation regulating these toxic compounds release leads to the development of various efficient technologies for their removal from wastewater. The most common treatment processes used include chemical precipitation, ion exchange, reverse osmosis, solvent extraction and adsorption. Among all these methods, adsorption process using sorbents, such as activated carbon, is one of the most popular ways since proper design of the adsorption process will produce high metal ions adsorption capacities. Each process presents its advantages and disadvantages, but in the past few years, extensive research has been undertaken to develop alternative and economic adsorbents containing natural polymers. Polysaccharides such as chitin [2,3] and starch [4,5] and their respective derivatives chitosan [6] and cyclodextrins [7] retain attention of scientific community. These biopolymers represent an interesting and attractive alternative as sorbents because of their specific structure, physico-chemical characteristics, chemical stability, high reactivity and excellent selectivity resulting from the presence of chemical reactive groups (hydroxyl, acetamido or amino functions) in polymer chains. Moreover, these products are abundant, renewable, biodegradable and are able to physically and chemically bind a wide range of molecules [8,9].
Agricultural by-products valorization as biosorbents for heavy metal removal also offers a potential alternative to existing processes and is yet subject to extensive studies. As example, agricultural biosorbents including soybean hulls, peanut hulls, almond hulls, cottonseed hulls and corncobs, have shown their ability to remove heavy metal ions from aqueous solutions [10-12].
In this review, the use of sorbents containing polysaccharides has been investigated as substitutes for current conventional methods used for removing metallic ions from solution. Data obtained using agricultural by-products and native or chemically modified carbohydrates will be described. Reported adsorption capacities will be indicated when possible to give an idea of sorbent effectiveness. Interactions between metallic ions and polysaccharides, metal ions complexation mechanism and adsorption models are also discussed on a theoretical point of view. Finally, this review presents a critical analysis of polysaccharide-based materials used in wastewater treatment and the advantages of natural biosorbents regarding classical adsorbents.
The affinity of metal ion toward solid surface or soluble chelating compound depends on numerous factors including the nature of sorbent and pollutant, pH, ionic force in bulk solution and temperature. In literature, various mechanisms are mentioned for metallic ions binding such as ion exchange, complexation or physical adsorption, that may create confusion [13]. In the case of metal ions, the term adsorption seems to be imprecise or vague, adsorption phenomenon referring to mass transfer and accumulation on solid surface.
The first research works carried out to elucidate the binding mechanisms of metal ions on solid surfaces were focused on interactions between metals and metal oxide surfaces because this phenomenon takes place in various scientific domains like geochemistry, catalysis and transport of compounds in natural or fresh water. These mechanisms can be described with classical theory of coordination chemistry. Solid particles surface or dissolved molecules in water are generally composed of ionic functional groups, negatively or positively charged. This phenomenon implies the existence of an electrical field that locally changes water properties in the solvation layer of the surface and the ion activity within this layer. According to interaction forces between the adsorbed molecule and the sorbent, complexes of external sphere or internal sphere are formed, corresponding to physical and chemical adsorption processes, respectively.
Complexes of external sphere
In this case, metal ions are attracted to solid surface by nonspecific bindings. These interactions are electrostatic in nature, (Figure 1) the internal sphere of hydration of cationic metals is not modified. For example, copper ions form complexes of external sphere with active surface or molecule by hydrogen bindings between surface functional groups and their own hydration sphere. Due to non-specific character, complexation of metal ion in external sphere does not lead to zero point of charge modification. So, it is possible to define ion exchange mechanism as the replacement of a metal ion initially complexed in external sphere with an active surface site by another metal having higher affinity. Many authors consider the analogy between complexation in external sphere and ion exchange, these mechanisms suggesting electrostatic interactions in nature [14].
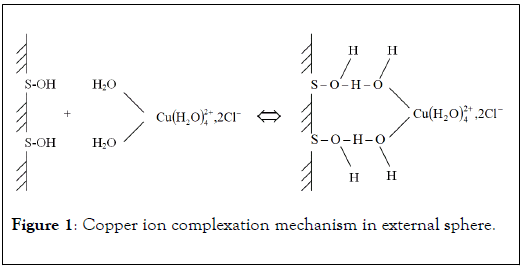
Figure 1: Copper ion complexation mechanism in external sphere.
Complexes of internal sphere
Penetration of metallic ion coordination sphere by chelating molecules or surface functional groups lead to chemical adsorption processes and complexation in internal sphere (Figure 2). In this case, it is about specific adsorption of metal in which hydration sphere is affected. This complexation mechanism can take place against electrostatic forces and at lower pH values than zero point of charge. The easily hydrolysable elements, such as Cu2+, Pb2+ or Hg2+, are often complexed by specific way. The specific binding of positively charged metal ions on solid surface or functional groups of active molecules modifies the zero point of charge value of the complexing material.
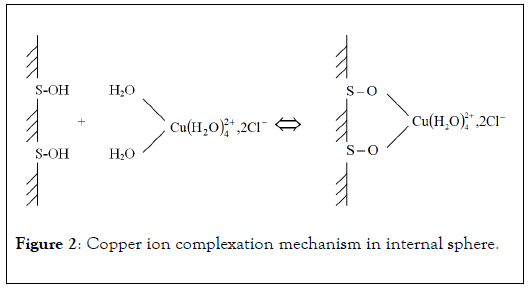
Figure 2: Copper ion complexation mechanism in internal sphere.
Whatever the complexation mechanism, numerous experimental parameters have a direct influence on metal removal efficiency. These major parameters that affect adsorption efficiency have to be specified and controlled.
Influence of pH
The pH value is the most important parameter that influences binding abilities of metal ions. Due to various pH values fixed in research works proposed in literature, comparison of metal ion adsorption efficiencies onto sorbents is particularly difficult. Moreover, to avoid overestimation of adsorption capacities, metal precipitation in aqueous solution has to be avoid by fixing pH at lower value than precipitation level (in general at pH value of 6).
The pH of solution determines hydrolysis rate of metal ion, his solubility and surface charge of sorbent. Moreover, a narrow pH range of one or two pH units exists, in which metal ion complexation rate suddenly increases whatever the sorbent is. The increase of pH leads to surface ionisable functional group dissociation and this dissociation phenomenon increases negative surface charge and attracts metal ions from solution. Moreover, competition of metal cations with protons for identical complexation sites is significantly affected with increasing pH value.
Influence of ionic force
The impact of ionic force on metal complexation cannot be generalized for all sorbents. The mechanism implied in metal ion removal governs the ionic force influence. Research works show the possibility of complexation mechanism to be identified by observation of ionic force influence on adsorption process [15]. A negligible influence of ionic force on adsorption capacities would involve complexation in internal sphere. Complexation in external sphere mechanism, due to the presence of electrostatic interactions, is sensitive to ionic force variations of aqueous solution.
Influence of temperature
Few studies about the influence of temperature on metal ion complexation onto biomaterials are reported in literature. It is due to the weak influence of temperature on biosorption process in the range 20°C-35°C [13]. However, Ozer et al. have shown the exothermic character of adsorption reactions of chromium (III) onto sugar beet pulp pectin [16].
So, numerous parameters directly determine the efficiency of metal ion removal onto biomaterials: pH of aqueous solution, ionic force and quality of effluent. These parameters have to be correctly identified and controlled to put in concrete form biosorption processes.
The study of saccharides behavior vs metallic ions aqueous solution has emerged in 1970’s [17-19] and is actually a major source of concern. On industrial applications point of view, their chelating properties can be useful for removal of mineral pollutants from wastewater [17].
These authors use theory of complexation mechanism to describe interactions between metal ions and mono- or polysaccharides. The formation of these complexes strongly depends on various parameters, such as linear density of charge of the polymer, nature of charged functional groups, metal chemistry, ionic force and wastewater pH value. These separation processes are governed by chemical equilibrium between complexation sites and ionic compounds in solution.
Interactions between metal ion and neutral mono- or polysaccharides
The studies carried out on complexes formation between metallic ion and hydrosoluble saccharides have been reviewed by Rendleman [18,19] and more recently by Angyal [20], Bailey et al. [2] and Demirbas [21]. These complexes have been identified by electrophoresis, NMR, FTIR spectroscopy, potentiometric titration, cyclic voltammeter. The advantage of these methods is their high sensitivity. For example, in electrophoresis, migration of saccharide associated with metal ion to cathode shows the existence of complex and his electrophoresis mobility is related to complex stability. Among the numerous polyols examined, cis-inositol (Figure 3), which presents the more important migration, is the chelating saccharide reference.
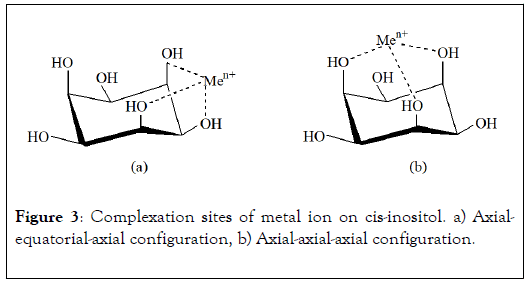
Figure 3: Complexation sites of metal ion on cis-inositol. a) Axialequatorial- axial configuration, b) Axial-axial-axial configuration.
All the mono or polysaccharides presenting almost two hydroxyl groups in axial position develop electrophoresis mobility. Mono and polysaccharide having exclusively hydroxyl groups in equatorial position, like glucose molecule, do not seem to form complexes with metal ions or in very low amount (lower than 1% of complexes in solution). In aqueous solution, one hydroxyl function cannot be in competition with water molecule that coordinates the metal ion. Solely favourable arrangement of two or three OH groups allows metal ion complex to be formed.
As revealing example, cis-inositol molecule contains six OH functions in exclusively axial-equatorial-axial positions sequence. Three of these hydroxyl groups are always located in syn position, i.e. dispersed on the same side with regard to the cycle. So, inositol presents two kinds of metal ion coordination site. The axial-equatorial-axial configuration was one of the first clearly identified complexation site by NMR and X ray analysis. This hydroxyl functions arrangement seems to be the most favourable for complex formation (Figure 3a). The three oxygen atoms of OH groups are equidistant to complexed ion. Due to steric effect, this site configuration is more favourable in the case of important ionic radius of metal, in particular when atomic radius is higher than 0.8 Å. If one of the three hydroxyl function is replaced by methoxyl group, complexation as well takes place but in lower proportion [20]. Metal ion with ionic radius lower than 0.8 Å are preferentially linked to axial-axial-axial complexation site (Figure 3b). However, this second OH functions arrangement is rare in natural polysaccharides.
These preferential sites have been observed and confirmed afterward, for monosaccharide like α-D-allopyranose [18]. In this case, Ca2+ ion, which ionic radius is equal to 1 Å, is coordinated according to a-e-a configuration of OH groups (Figure 4a). In the case of furanose cycle, the optimal complexation site of calcium ion is arranged with three hydroxyl functions in cis-cis configuration (Figure 4b) and hydroxyl groups form equilateral triangle of 2.8 Å of side. More generally, stability of complexes decreases when site configurations vary among this sequence: three OH in e-a-a configuration of pyranose cycle>three OH cis-cis of furanose cycle>two OH cis-cis of furanose>two OH cis-cis of pyranose>two OH of furanose.
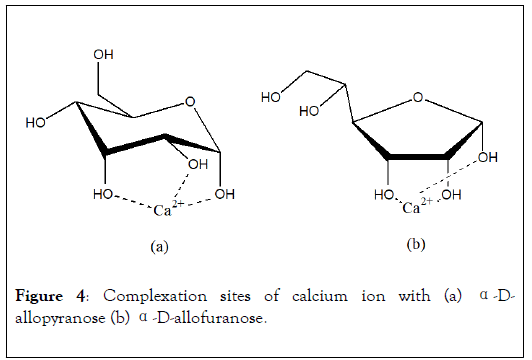
Figure 4: Complexation sites of calcium ion with (a) α-Dallopyranose (b) α-D-allofuranose.
Except peripheral hydroxyl functions, implication of oxygen atoms of saccharide cycle in metal complexation mechanism is also possible and has been already observed.
In the anhydro-β-D-glucopyranose, NMR study has shown the implication of O2, O4 and O5 oxygen atoms [20] (Figure 5). In the case of monosaccharide, a maximum of three OH groups can react with metal ion as chelating agent. When polysaccharide is used as sorbent, more than three OH groups can form interactions with metal.
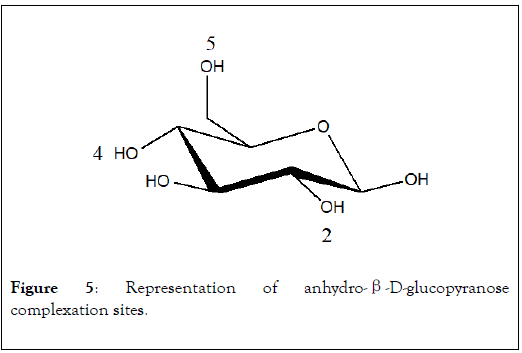
Figure 5: Representation of anhydro-β-D-glucopyranose complexation sites.
The chemical environment of binding sites is also an important parameter. For example, Jezowska-Bojczuk et al. [22] estimate that in natural water systems, neutral saccharides have very low affinity towards most of common metal ions. It is due to important competition between hydroxyl groups of saccharides and water molecules for identical metal ions complexation. The presence of ionizable chemical groups on carbohydrates, such as carboxylic functions, would contribute to promote metallic ions binding mechanism in biological systems.
Interactions between metal ion and anionic mono- or polysaccharide
In pectic substances of biomass, the polymer of galacturonic acid is a helical three dimensional structure of 1.34 nm (0.435 nm in projection) for each monomer [23]. This chemical structure is relatively open and has low steric effect for interaction with metal ions. In biological systems, carboxylic functions of this polymer develop affinities with most of metallic ions. Structure of calcium-galacturonic acid complex as proposed by Rendleman (Figure 6).
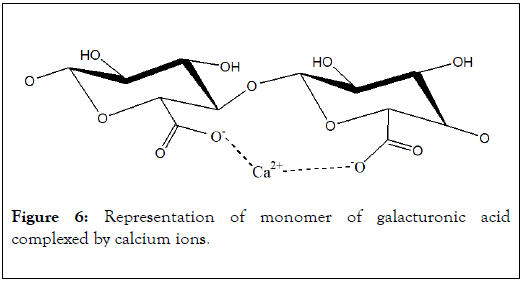
Figure 6: Representation of monomer of galacturonic acid complexed by calcium ions.
Deiana et al. have studied complexation mechanism of Mn2+, CO2+ and Ni2+ ions by galacturonic acid and the authors conclude that two carboxylate functions are implied in metal ion binding [24]. The complexation mechanisms of calcium ions on polygalacturonic acid in aqueous solution have been also specifically studied by Manunza et al. [25,26]. When calcium ions are added in the solution, dimerization of polymer occurs followed by an aggregation step of monosaccharide chains. In 1995, Jezowska-Bojczuk et al. [22] carried out potentiometric and spectroscopic study on copper ion removal by polymer of galacturonic acid which confirmed that carboxylic functions were Cu2+ chelating agent.
Interactions between metal ion and carboxylate functions
In 1988, Carell et al. have published research work on binding selectivity of numerous metallic ions for electron doublets pairs of oxygen atom from carboxylate group [27]. This study dealt with the identification of metal ion preference towards two free electron pairs in syn and anti-position of oxygen atom and the evaluation metal-oxygen distance. Carboxylate function has a delocalized negative charge in which each oxygen atom possesses two electronic pairs oriented with an angle of 120° C with respect to C-O link and carboxylate plane (Figure 7a). The electronic pair in syn position is located in the same side than the two oxygen atoms in contrast with anti-electron pair which the angle becomes negative (Figure 7b). The direct link shows metal complexation with the two oxygen atoms of carboxylate function that implies metallic ion position at an angle contained between 80 and 90°.
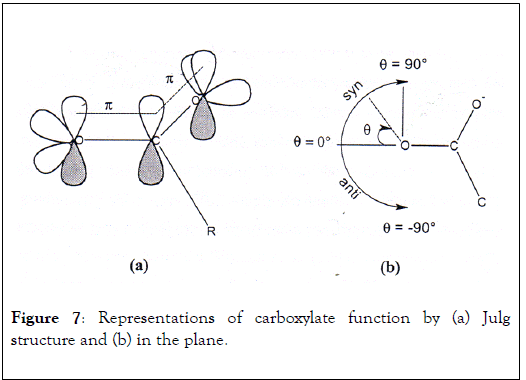
Figure 7: Representations of carboxylate function by (a) Julg structure and (b) in the plane.
Adsorption models
Adsorption is a surface phenomenon resulting from mass transfer between gaseous or liquid phase and solid phase. This mass transfer takes place on several steps:
• Diffusion of solute from fluid to boundary layer,
• Mass transfer from film to sorbent (film diffusion or external transport),
• Diffusion of compounds from sorbent surface to inside porosity if the adsorbent is a porous material in nature (intraparticle diffusion),
• Fixation of compound on surface active sites.
This mass transfer phenomenon results from fluid-adsorbent interactions of two types:
• Physical, when fluid-adsorbent interactions are low from energy point of view (Van der Waals forces up to 100 kJ mol). This mechanism, which is generally fast, does not modify adsorbed molecule structure and is reversible in nature.
• Chemical, when covalent links between sorbent surface and adsorbed molecule are formed. The molecule undergoes a modification of its chemical properties. This chemical adsorption mechanism is generally not reversible.
Langmuir model
The Langmuir model traduces the thermodynamically equilibrium between adsorbed quantity and free concentrations of adsorbed molecule/sorbent couple [28]. This model is based on the following hypothesis.
• Maximum adsorption corresponds to complete monolayer coverage of adsorbent surface.
• Adsorption sites are homogeneous in nature with constant adsorption energy.
• The adsorbed molecules don ’ t develop interaction among themselves.
• Langmuir equation is:
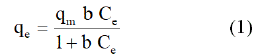
Where qm (mol.g-1) is the maximum adsorption capacity corresponding to complete monolayer coverage, Ce (mol.l-1) the equilibrium solute concentration and b (l.mol-1) the equilibrium constant related to energy of sorption.
Feundlich model
The empirical Freundlich equation considers adsorption energy variations with adsorbed quantity evolution. This interaction energies distribution is explained by heterogeneity of adsorption sites [29]. Freundlich model does not allow adsorption limit but considers the existence of interactions between adsorbed molecules and is written as:

Where K and n are model constants.
Extensive Langmuir model
The extensive Langmuir model, initially developed by Butler and Okrent [30], is used to describe adsorption isotherms in multi-compound systems. Model hypothesis are identical to Langmuir model in mono-compound system. The adsorption capacity of solute, i, in a mixture of N compounds is:
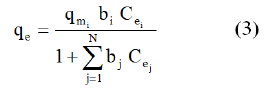
Where qmi and bi are Langmuir parameters obtained in monocompound system for solute i.
Complexation models
Complexation models allow extending association models between ions in aqueous solution to active chemical functions that compose polysaccharides. Several models have been proposed in literature to describe adsorption phenomenon at liquid-adsorbent interfaces and all are based on identical following hypothesis:
• Adsorbent is composed of well identified specific functional groups. These localized reactive sites react with ionic solute to form complexes by covalent links or as ions pairs.
• Law of mass action can describe ionization and complexation equilibrium.
• Surface charge and electric potential result from protonation and functional groups complexation reactions. This electric field and electrostatic effects are taken into account by a correction factor added to thermodynamic constants. This term can be calculated from electric double layer theory.
Functional groups present protonation/deprotonation reactions. For example, in the case of carboxylic functions this equilibrium is:
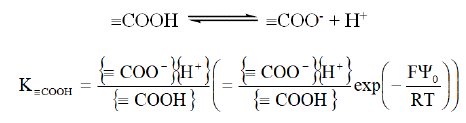
With F Faraday constant (96 485 C.mol-1), T temperature (K), R universal gas constant (8.314 J.mol-1.K-1) and Ψ0 surface potential (V).
In the same way, complexation equilibrium of metal ion Me2+ with carboxylic group is:
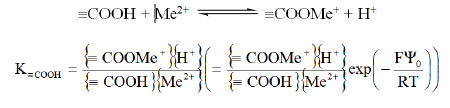
This equilibrium implies competition between metal ion Me2+ and protons H+ in solution.
Among the numerous wastewater treatment ways dedicated to metal ions removal, adsorption process is widely used. Classical adsorbents consist in ion-exchange resins, activated carbon powder and fibers, and, more rarely, agricultural by-products. A review of biosorption processes shows that water treatments systems carried out with biomass composed of polysaccharides seems to be attractive and effective.
Chitin and chitosan
Chitin is a structural polymer from arthropods exoskeleton and cephalopods endoskeleton. This polymer is a long-chain of an Nacetyl- D-glucosamine, a derivative of glucose (Figure 8). Chitosan is composed of D-glucosamine and N-acetyl-Dglucosamine units linked in β(1→4) and obtained by partial Ndeacetylation of chitin. Numerous studies on chitin and chitosan valorization in wastewater treatment for metal ions and dyes removal are reported in literature. These materials are very efficient chelating agent towards metals and especially Pb2+, Cd2+ and Cu2+ as shown in Table 1. Due to various free amine groups, chitosan allows higher adsorption capacities than chitin. Chitosan can be packaged in powder, fibers, pellets, membranes or gel [31]. As a powder, chitosan has a high exchange surface but leads to an important pressure head in column when used as stationary phase. The high solubility of chitosan in acidic environment avoids regeneration by acidic solution. Packaging in gel improves intra-particle diffusion by favoring active sites accessibility. Chitin and chitosan possess several other advantages and characteristics that make them excellent materials for industrial use.
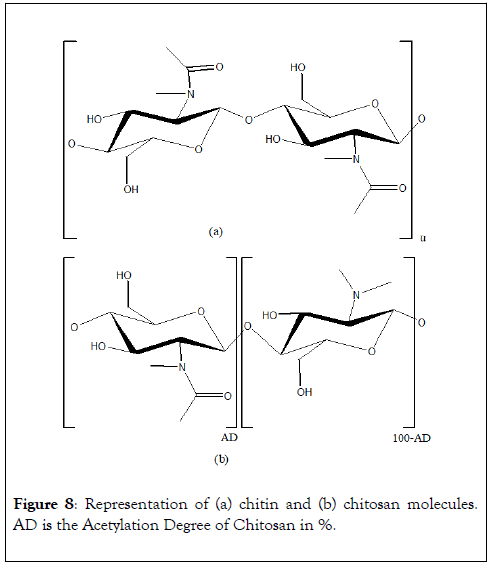
Figure 8: Representation of (a) chitin and (b) chitosan molecules. AD is the Acetylation Degree of Chitosan in %.
| Adsorbent | Characteristics | Metal-experimental conditions | Adsorption model and capacities | Affinity sequence | Reference |
|---|---|---|---|---|---|
| Chitin | granular 0.2-6.3 mm |
[chitin]=2 g l-1 24 hours Cd2+ |
Langmuir (qm mmol g-1; b l mmol-1) qm=0.131; b=0.122 |
[32] | |
| Chitin of shrimp | Pb2+ Cd2+ Cu2+ Zn2+ |
Freundlich (K mg g-1) K=926 K=35 K=42 K=11 |
Pb2+>Cu2+> Cd2+>Zn2+ |
[33] | |
| Chitin of crab | Pb2+ Cd2+ Cu2+ Zn2+ |
K=265 K=7 K=17 K=23 |
Pb2+>Zn2+> Cu2+>Cd2+ | ||
| Chitin of crayfish | Pb2+ Cd2+ Cu2+ Zn2+ |
K=4 K=19 K=204 K=11 |
Cu2+>Cd2+> Zn2+>Pb2+ | ||
| Chitosan | Pellets | T=25° C; 48 h [chitosan]=1 g l-1 Cu2+ Zn2+ Ni2+ |
qe mmol g-1 qe=2.5 qe=0.9 qe=0.8 |
Cu2+>Zn2+> Ni2+ | [34] |
| Powder 0.25-0.5 mm |
T=25° C 24 hours pH 6 [U(VI)]0=140 mg l-1 U(VI) |
qe mg g-1 qe=450 | [35] |
Table 1: Metal ions adsorption capacities of chitin and chitosan.
Agricultural by-products
Faced with the increase of wastewater amounts and more precise knowledge on heavy metal toxicity, more and more stringent standard set are fixed by European Union. Moreover, quality and preservation of environment must not lead to too important cost for concerned industries. So, innovative processes using abundant and low-cost biomass (microorganisms, agricultural by-products) seem to be economically attractive. Adsorption of metal ions onto these various biomaterials is mainly due to complexation mechanism with surface chelating agents, essentially polysaccharides.
Many research works deal with adsorption capacities of microorganisms because this biomass is generated by industrial fermentation processes (pharmaceutical products, enzyme production) [13]. As reported by Volesky [36], several bacteria, fungus and algae develop important biosorption capacities but are not detailed in this review.
Since last two decades, biosorption has emerged as a potential and cost-effective option for heavy metal removal from aqueous solution using agricultural biosorbents. Several data of literature clearly show the potential of these natural biopolymers in wastewater treatment. Research works are focussed, since few years, on optimization and economical studies to evaluate feasibility and cost effective potential of biosorption processes with regards to existing wastewater treatment [37-39]. Table 2 shows a non-exhaustive list of selected examples of agricultural by-products application in biosorption and corresponding adsorption capacities of metal ions (mainly Ni2+, Cu2+, Pb2+, Zn2+, Cd2+ and Cr(III)). This review shows interesting adsorption capacities of various agricultural by-products. Even if these “native” biomaterials seems to be attractive for wastewater treatment processes, several research works are carried out to increase their adsorption capacities, especially by chemical treatments. Cellulose-based materials, such as wood peels and sawdust, have low complexation capacities towards metal ions due to their low surface functional groups content. In this case, innovative researches have been considered to increase their efficiency by chemical modifications of lignocellulosic materials. The most efficient chemical treatment is phosphorylation reaction using phosphoric acid solution according to Fiset et al. [40]. Marshall et al. suggest sodium hydroxide treatment of peanut, sugar cane, hazelnut, rice, cotton, maize, almond and walnut, followed by citric acid treatment, to increase metal ions adsorption capacities. Moreover, for economical point of view, chemical modifications of biosorbents have to be minimized during wastewater treatment process set up.
| Adsorbent | Metal-experimental conditions | Adsorption model and capacities | Affinity sequence | Reference |
|---|---|---|---|---|
| modified soya seed (NaOH/ citric acid) | pH=4.8 [adsorbent]=10 g l-1 Ni2+ Zn2+ Cd2+ Pb2+ Cu2+ |
Langmuir (qm mmol g-1; b l mmol-1) qm=1.03; b=0.858 qm=1.09; b=0.705 qm=1.21; b=0.281 qm=1.68; b=0.168 qm=1.76; b=0.384 |
Cu2+ » Pb2+> Cd2+>Zn2+> Ni2+ | [11] |
| Shell of: soya, almond, cotton, peanut |
Cu2+ Cu2+ Cu2+ Cu2+ |
qe mmol g-1 qe=1,5 qe=1,23 qe=1,02 qe=1,06 |
Soya>almon> peanut>cotton | |
| Modified wastes (NaOH) | pH=4.8; 24 hours [adsorbent]=10 g l-1 [Cu2+]=20 mmol l-1 Sugar cane Peanut shell Hazelnut shell Rice bran Cotton shell Maize waste Soya shell Almond shell Walnut shell |
qe mmol g-1 qe=0.17 qe=0.14 qe=0.58 qe=0.32 qe=0.51 qe=0.18 qe=0.55 qe=0.67 qe=0.40 |
almond>hazelnut>soya>cotton>walnut>rice>maize » sugar cane » peanut | [10] |
| Modified wastes (NaOH/ citric acid) | pH=4.8; 24 hours [adsorbent]=10 g l-1 [Cu2+]=20 mmol l-1 Sugar cane Peanut shell Hazelnut shell Rice bran Cotton shell Maize waste Soya shell Almond shell Walnut shell |
qe mmol g-1 qe=1.27 qe=0.83 qe=1.12 qe=0.76 qe=0.98 qe=0.99 qe=1.44 qe=1.31 qe=0.31 |
Soya>almon>sugar cane>hazelnut> maize » cotton>peanut>rice>walnut | [10] |
| Rice waste | [adsorbent]=5 g l-1 Cu2+ Cr(III) Ni2+ Pb2+ |
Langmuir (qm mmol g-1) qm=0.033 qm=0.100 qm=0.031 qm=0.039 |
Cr(III)>Pb2+ » Cu2+ » Ni2+ | [42] |
| Rice waste modified by EDTA/NaOH | Cu2+ Cr(III) Ni2+ Pb2+ |
qm=0.139 qm=0.184 qm=0.149 qm=0.138 |
Cr(III)>Ni2+> Cu2+ » Pb2+ | |
| Hop stems: native hydrolysed esterification |
T=20° C, 24 hours [Pb2+]=0.3 mmol l-1 Pb2+ |
qe mmol l-1 qe=0.259 qe=0.273 qe=0.051 |
[43] | |
| Hop leafs: native hydrolysed esterification |
Pb2+ | qe=0.259 qe=0.273 qe=0.051 |
||
| Treated wood sawdust Mangifera indica |
T=25° C, pH=6 Sawdust 100 µm [Cu2+]=2.7 mmol l-1 Cu2+ |
qe mmol l-1 qe=0.259 | [44] | |
| Treated wood sawdust Abies magnifica | T=27° C, pH=5.5 60 g l-1 sawdust 2 mm Cu2+ Cr(VI) |
Langmuir (qm mmol g-1) qm=0.112 qm=0.194 |
[45] | |
| Treated straw (acid/NaOH) | pH=4-7 10 g l-1 treated straw [Cu2+]=50-250 mmoll-1 [Cd2+]=50-250 mmol l-1 |
Langmuir (qm mmol l-1) qm=0.18 qm=0.13 | [46] | |
| Sugar beet pulp pectin | [pulp]=20 g l-1 [Me2+]=0.1-10 mmol l-1 Ni2+ Zn2+ Cd2+ Pb2+ Cu2+ CO2+ |
Freundlich (K mg1-1/n l1/n g-1) K=0.158; 1/n=0.95 K=0.063; 1/n=0.82 K=0.146; 1/n=1.05 K=0.400; 1/n=3.62 K=0.435; 1/n=2.06 K=0.224; 1/n=1.03 |
Pb2+>CO2+> Cu2+>Ni2+> Zn2+>Cd2+ | [47] |
| Modified sugar beet pulp (NaOH/Citric acid) | pH=5, T=25° C [pulp]=1.25 g l-1 [Cu2+]=5-25 mmol l-1 |
Langmuir (qm mg g-1; b l mg-1) qm=119; b=0.039 |
exothermal character of adsorption reaction | [48] |
| Saponified sugar beet pulp | T=25° C, pH=7 [NaNO3]=0.1 mol l-1 [pulp]=14.55 g l-1 Ca2+ Ni2+ Zn2+ Cd2+ Pb2+ Cu2+ |
Langmuir (qm meq g-1; b l meq-1) qm=0.83; b=0.185 qm=0.97; b=0.505 qm=1.01; b=1.241 qm=1.11; b=1.320 qm=1.28; b=2.930 qm=1.35; b=3.740 |
Cu2+ » Pb2+> Zn2+ » Cd2+>Ni2+> Ca2+ | [49] |
| [pulp]=14.55 g l-1 Ca2+ Ni2+ Zn2+ Cd2+ Pb2+ Cu2+ |
Freundlich (K mg1-1/n l1/n g-1) K=2.54; 1/n=0.35 K=3.54; 1/n=0.34 K=1.56; 1/n=0.12 K=1.48; 1/n=0.10 K=2.21; 1/n=0.14 K=2.76; 1/n=0.16 |
|||
| Sugar beet pulp pectin | pH=7 [NaClO4]=0.1 mol l-1 [pulp]=20 g l-1 [Cr(III)]=5-30 mg l-1 T=20° C T=30° C T=40° C T=50° C |
Freundlich (K mg1-1/n l1/n g-1) K=0.369; 1/n=0.594 K=0.269; 1/n=0.615 K=0.195; 1/n=0.613 K=0.14; 1/n=0.647 |
exothermal character of adsorption reaction | [16] |
| Cr (III) de 5-30 mg/l T=20° C T=30° C T=40° C T=50° C |
Langmuir (qm mg g-1; b l mg-1) qm=1.919; b=0.206 qm=1.814; b=0.140 qm=1.569; b=0.104 qm=1.493; b=0.073 |
Table 2: Review of experimental studies on metal ion adsorption onto native or modified agricultural by-products.
Preliminary experiments concerning biosorption of copper, zinc and lead onto chicory pulp [41] have been also carried out in batch reactors in our laboratory. Chicory pulp mainly contains cellulose, lignin, pectin and inulin. The results show promising adsorption capacities, between 50 and 100 mg.g-1. We also studied identification and characterization of inulin-metal ions complexation phenomenon in aqueous solution.
Innovative studies on metal ions removal by pectic-based materials show their influence on adsorptions capacities of sugar beet pulp [48-51]. Dronnet et al. have observed citrus and sugar beet pulps affinity with cations according to following the sequence: Cu2+ ≈ Pb2+>Zn2+ ≈ Cd2+>Ni2+>Ca2+. Kartel et al. [47] have also observed high affinities for Cu2+, Pb2+ and CO2+, lower adsorption capacities for Ni2+ and no affinity towards Zn2+ and Cd2+. More recently, Reddad et al. [50,51] have shown that equilibrium data, resulting from batch adsorption studies, fitted well with Langmuir model and maximum adsorptions capacities ranged from 0.202 to 0.356 mmol.g-1 with the following affinity order: Pb2+>Cu2+>Zn2+>Cd2+>Ni2+ ≈ Cr(III). Due to their important metal ion adsorption efficiency, even at very low concentration level, Kartel et al. [47] suggested medical application of pectin to treat patients being poisoned or exposed to metallic pollution. In term of binding mechanism, Dronnet et al. [49] and Reddad et al. [52], highlighted ion exchange mechanism with Ca2+ ions contained in native pulps. Concerning Pb2+ and Cu2+ removal, their adsorption capacities exceed cationic exchange capacity of pulps, which would imply the existence of a complementary complexation mechanism. Due to important hydration properties of native pulp, Dronnet et al. [49] have proposed a modification of raw and saponified pulp by crosslinking reactions using formaldehyde, epichlorohydrin to improve their mechanical properties. Crosslinking reaction onto saponified pulp by epicholorohydrin seems to give more promising results.
Recently, sugar cane bagasse, maize corn cob and jatropha oil cakes were used for efficient removal of chromium and nickel under optimized conditions [53,54]. And, finally, NaOHpretreated rose petals, calcium-treated sargassum and sugarcane modified with succinic anhydride have also been tested for significant removal of lead [55-57].
Currently, several research works have been carried out for the development of cheaper and more effective adsorbents containing natural polymers. Among these, polysaccharides such as chitin and starch, and their derivative (chitosan and cyclodextrin, respectively) deserve attention [2,6]. These biopolymers represent an interesting and attractive alternative to classical adsorbent because of their specific structure, physiochemical characteristics, chemical stability, high reactivity and selectivity towards aromatic compounds and metals, resulting from the presence of chemical reactive groups (hydroxyl, amine and carbonyl) in polymer chains. It is also well known that polysaccharides have the ability to physically and/or chemically interact with a wide range of molecules [8]. This review only present few data obtained using chitin, chitosan, pectin, starch and cyclodextrin derivatives, which are the more popular saccharide-based materials, studied since few years.
The excellent adsorption ability of these polysaccharides towards metal ions is mainly attributed to high hydrophilic behavior of polymers due to hydroxyl groups of glucidic units, presence of highly chemically reactive functional groups, and flexible structure of polymer chain. One of the most important and useful feature of polysaccharide, especially chitin and starch, is their chemical reactivity. They possess a large number of reactive functions (hydroxyl and/or acetamino groups) present at the -2,-3 and -6 positions in the glucosidic unit (Figure 9). These groups allow direct substitution reactions (esterification or etherification reactions) or chemical modifications (hydrolysis, oxidation or grafting reactions), usually referred to as chemical derivatization, yielding different polysaccharide derivatives for specific domains of applications.
Figure 9: Representation of amylose molecule contained in starch.
Starch and chitin derivatives could be classified in three main classes of polymers:
• Modified polymers such as cationic starches, carboxymethylchitin.
• Derivatized biopolymers, including chitosan and cyclodextrin derivatives.
• Polysaccharide-based materials such as resins, gel, membranes, composite materials.
Many chitosan-based sorbents have been tested for their pollutant binding ability, under various conditions. For example, Wan Ngah et al. [58] have investigated the removal of copper ions from aqueous solutions using crosslinked chitosan beads. Chitosan-based materials were prepared using glutaraldehyde, epichlorohydrin and ethylene glycol diglycidyl ether and the uptakes on these chitosan derivatives were respectively: 59.6, 62.4 and 45.9 mg of Cu2+ per g of beads. The possible structures of various crosslinked chitosan beads (Figure 10).
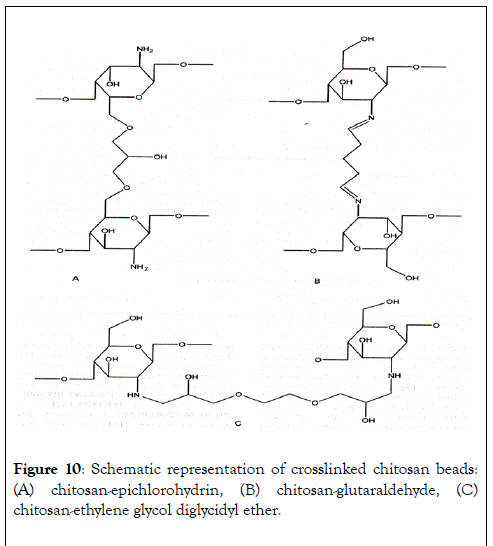
Figure 10: Schematic representation of crosslinked chitosan beads: (A) chitosan-epichlorohydrin, (B) chitosan-glutaraldehyde, (C) chitosan-ethylene glycol diglycidyl ether.
The metal adsorption process was highly pH dependent and the beads could be easily regenerated. Ruiz et al. [59] proposed the use of glutaraldehyde-chitosan beads as effective chelating resins for removing palladium from dilute solutions. Their identified that sorption kinetics are controlled by particle size, crosslinking ratio and palladium concentration. Heavy metal adsorption onto crosslinked chitosan beads has been also studied by Juang and Shao [34]. They showed that adsorption capacities depend on the nature of the crosslinking agent. The adsorption increased with increasing pH of the solution, due to the competitive adsorption of proton and metals.
Research works using chitosan derivatives were also carried out by Guibal and co-workers [60-64]. They reported that crosslinked chitosan beads can be used for the removal and the recovery of toxic or valuable precious metals such as arsenic, palladium, osmium, iridium and gold. For example, chitosan beads containing sulfur groups were very efficient to remove gold from dilute acidic solutions: maximum adsorption capacity reaches 600 mg of Au per gram of chitosan beads. They also introduced a new procedure to produce molybdate-impregnated chitosan sorbent. The impregnation was performed by direct coagulation in a molybdate bath. These modified beads were successfully tested for As(III) and As(V) removal from solutions. Finally, Lee et al. [65] proposed the use of spherical chitosantripolyphosphate chelating resins as sorbents for the removal of copper ions. The maximum adsorption capacity at pH=5 was about 200 mg of Cu2+ per gram of beads at an initial concentration of 1000 ppm of copper ions.
Copello et al, [66] have investigated the generation of layer-bylayer silicate-chitosan composite biosorbent. The film was evaluated for its capability in the removal of Cd(II), Cr(III) and Cr(VI) from aqueous solutions. Langmuir and Freundlich equations fit results of adsorption isotherms. Maximum adsorption capacities reached 0.46 and 0.89 mmol.g-1 for cadmium and chromium ions respectively. Adsorption and desorption characteristics of mercury ions using aminated chitosan beads have been investigated by Jeon and Park [67]. They conclude that adsorption of mercury was an exothermic process. The beads were not greatly affected by ionic strength, organic compounds and alkaline earth metal ions. Mercury ions adsorbed on aminated chitosan bead were desorbed at 95% yield by EDTA and the adsorption capacity of the recycled beads can still be maintained at 90% level at 5th cycle.
Increasing recent attention is also given to other carbohydrate polymers such as starch and cyclodextrin derivatives. Ciesielski and Tomasik [68] have studied complexation mechanism of starch polysaccharides (amylose and amylopectin) with metal cations (Cu2+, Fe3+, Mn2+ and Ni2+) by Electron Paramagnetic Resonance spectroscopy and conductivity measurements. They concluded that hydroxyl groups of polysaccharides (D-glucose units) are coordination sites. Zhang and Chen [69] proposed crosslinked starch graft copolymers containing amine groups. The polymer, used as support for lead and copper ion sorption, were obtained by grafting dimethylaminoethyl methacrylate onto commercial starch. The authors observed that two hours of mixing allows adsorption equilibrium to be reached with significant adsorption capacities. Adsorption phenomenon for metal ions is influenced by pH value of solution because of protonation and deprotonation of amine groups on sorbent surface. POCl3 crosslinked carboxymethylstarch has been used by Kim and Lim [70] as biosorbent for divalent metallic ion complexation. Several hundred ppm of copper, lead, cadmium and mercury ions could be efficiently removed from aqueous solution by dispersing 1% mass of the modified starch within few minutes. Adsorption kinetics is fast and equilibrium adsorption capacities depend on carboxymethyl groups and starch content in the solution.
Few studies have also investigated the possibility for the use of extracellular polysaccharide as biosorbent in the removal of toxic heavy metals [71-74]. For example, Moon et al. [72] have examined the potential applicability of Pestan, a fungal extracellular polysaccharide that consists of galactosamine, glucuronic acid, galacturonic acid, glucose and galactose, in wastewater treatment. Batch sorption experiments were performed in synthetic wastewaters containing lead or zinc ions. The equilibrium adsorption capacities were shown to be 120 mg of Pb2+ per g of Pestan and 60 mg of Zn2+ per g of Pestan. The results obtained have shown that the extracellular polysaccharide sorption behavior depends on metal ion species and complexing intensities with metal ions. Unlike the Freundlich equation, the Langmuir equation was not suitable to well describe obtained sorption data. It is due to polysaccharide heterogeneity and cooperative interactions with its functional groups such as hydroxyl, amine and carboxyl groups.
Recently, new composite materials have been prepared to remove heavy metals from wastewater. Wan et al. [74] suggested a new hybrid adsorbent based on chitosan immobilized on sand particles. The sorption results indicated the possibility for chitosan-coated sand to be used in metal ion removal from contaminated groundwater. Alginate-chitosan hybrid gel beads were prepared by Gotoh et al. [75] for the first time and shown to very rapidly adsorb heavy metal ions. Steenkamp et al. [76] investigated the capacity of copper ions adsorption onto composite alumina/chitosan membranes. Equilibrium adsorption capacities were about 0.2 g Cu2+ per g chitosan. These hybrid materials are preferred due to their chemical stability as a function of pH. Finally, recent works of Dahiya et al. [77] on biosorption of lead and copper ions from aqueous solutions have shown important adsorption capacities of these compounds onto pre-treated crab shell biomass. At equilibrium, adsorption capacities were 19.83 and 38.62 mg.g-1 for lead and copper, respectively.
Advantages of using natural polymers for adsorption
Compared with conventional sorbents used for removing metal ions from aqueous solution, such as commercial activated carbon and synthetic ion-exchange resins, sorption using polysaccharide-based materials as biosorbent offers several advantages:
• The sorbents are low-cost materials obtained from natural raw resources. Most of commercial polymers and ion-exchange resins are derived from petroleum-based materials using processing chemistry that is not safe and environmentally friendly. Today, there is a growing interest in developing natural low-cost alternatives to synthetic polymers.
• The use of biosorbents is extremely cost-effective. Crosslinked materials are easy to prepare with relatively inexpensive reagents allowing low operating costs. For example, activated carbon and ion-exchange resins are quite expensive and the higher the quality, the greater the cost.
• The materials are versatile. This versatility allows sorbent to be used under different forms, from insoluble beads, to gels, films, membranes and filters. Materials are available in a large variety of structures with a variety of properties.
• Sorbents containing polysaccharides are very efficient for the removal of pollutants at different concentrations. They possess high capacity and high rate of adsorption, high efficiency and selectivity in depollution of either very dilute or concentrated solutions.
• With repetitive functional groups, biopolymers provide excellent chelating materials for a wide range of pollutants including dyes [78-80], aromatic compounds [81-83] and, as shown in this paper, numerous metal ions.
• Crosslinked materials, in particular cyclodextrin polymers, possess an amphiphilic character. It is this character of these sorbents that makes them so appealing, since they are enough hydrophilic to considerably swell in water allowing fast diffusion process for the adsorbate, while at the same time they possess highly hydrophobic sites which efficiently trap non polar pollutants. Activated carbons for example, poorly adsorb some hydrophilic substances and metal ions.
Limitations in adsorption by natural adsorbents
Of course, disadvantages in using polysaccharides in wastewater treatment appear and can be listed as follows:
• Adsorption properties of an adsorbent depend on the different sources of raw materials. The sorption capacity of chitin and chitosan materials depends on the origin of the polysaccharide the degree of N-acetylation, molecular weight and solution properties and varies with affinity for water, percent of deacetylation and amino group content. These parameters control the swelling and diffusion properties of the polysaccharide and influence its characteristics. These problems as well as heterogeneity of chemical composition can explain why it is difficult to transfer the use of polysaccharidebased materials from laboratory to industrial scale.
• The extreme variability of industrial and municipal wastewaters must be taken into account in the design of any polysaccharide system. Each type of pollutant may need its own specific polysaccharide. Each polysaccharide seems to have its specific application as well as inherent advantages and limitations in wastewater treatment. Chitosan-based materials have high affinities for heavy metal ions. Cyclodextrin sorbents have a remarkable capacity to form inclusion complexes with organic molecules, especially aromatics, but a lower affinity for metals.
• Performance strongly depends on the type of material used. The adsorption properties depend on the extent of chemical activation and modifications. In the case of chitosan, the best method of achieving selective extraction is to use a metal specific ligand, but it has proved impossible to find specific ligand for each metal ion.
• The efficiency of adsorption depends on physio-chemical characteristics such as porosity and specific surface area of sorbents. Another problem with insoluble polysaccharidebased materials is their poor physiochemical characteristics, especially porosity. Polysaccharides are, in general, non-porous and their derivatives have a low specific surface area. Dried pectin substances like sugar beet pulp develop very low specific surface area of about 5 m.g but their hydration and water retention capacity allow specific surface area of about 300 m.g to be obtained. Chitosan has also a low specific area ranging from 2 and 30 m.g. Glutaraldehyde-crosslinked chitosan beads, epichlorohydrin-cyclodextrin gels and epichlorohydrinstarch beads develop a specific surface area of 60, 213 and 350 m.g respectively. In comparison, most of commercial activated carbons develop between 800 and 2 000 m.g [84].
• Performance depends on wastewater characteristics. Metal ions complexation phenomenon is strongly pH dependent. The wastewater pH is an important factor in speciation of metallic ions. In addition, in strong acidic conditions, amine groups present in the chitosan beads easily form protonated groups, which induce an electrostatic repulsion of metal ions. This protonation induces a competition between protons and metal ions for adsorption sites.
The removal of polluting metal ions from aqueous effluents has received much attention in recent years. Due to increasing cost of conventional adsorbents, development of polysaccharide derivatives from renewable resources as sorbent is one of the most attractive technology to remove heavy metal from concentrated or diluted solutions. It is evident from literature review that polysaccharide-based materials have demonstrated important adsorption capacities comparable to commercial activated carbon or ion-exchange resins removal capabilities. The literature data show that the sorption capacity, specificity and adsorption kinetics are mainly influenced by chemical structure and composition of polysaccharide-based materials, and also by the accessibility of chelating and complexing groups.
Despite the number of research works published on natural adsorbents for metal ions removal from wastewater, only few reports contain a full study comparing various sorbents. Comparison of these low-cost sorbents and polysaccharide-based materials is difficult due to variability in data presentation and experimental conditions. So, additional works focusing on adsorption mechanism understanding are necessary and process development approach at an industrial scale must be investigated.
Citation: Joly N, Ghemati D, Aliouche D, Martin P (2020) Interaction of Metal Ions with Mono- and Polysaccharides for Wastewater Treatment: A Review. Nat Prod Chem Res. 8:373. DOI: 10.35248/2329-6836.20.8.373
Received: 26-Jun-2020 Published: 17-Jul-2020
Copyright: © 2020 Joly N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.