Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- The Global Impact Factor (GIF)
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 11, Issue 4
In Vitro Dissolution Study of Metronidazole Tablet in Different Fluids
Deeptipiya Baniya1, Kiran Bikram Bohara2, Dharma Pd. Khanal3, Saroj Bashyal4* and Priyanka Shahi52Corel Pharmaceuticals Pvt. Ltd, Manmohan Memorial Institute of Health Sciences, Tribhuvan University, Nepal
3Pharmacy Department, Manmohan Memorial Institute of Health Sciences, Nepal
4Quality Assurance Department, Vega Pharmaceuticals Pvt. Ltd, Manmohan Memorial Institute of Health Sciences, Nepal
5Yeti Heath Science Academy, Purbanchal University, Nepal
Received: 28-May-2020 Published: 29-Jun-2020, DOI: 10.35248/2153-2435.20.11.623
Abstract
Objective: The plot of this study is to obtain a grasp regarding the effect of different fluids (Gastric medium, simulated gastric fluid without enzyme, ORS solution, rice water, lentil soup, watermelon juice, apple juice, mango juice, pomegranate juice, black and green tea) on dissolution of metronidazole tablet by alteration of different medium.
Method: Our study had focused on the in vitro dissolution study of metronidazole in the presence of different fluids (Gastric medium, simulated gastric fluid without enzyme, ORS solution, rice water, lentil soup, watermelon juice, apple juice, mango juice, pomegranate juice, black and green tea) which has been commonly used in diarrheal condition to obtain quickest and profound dissolution for the metronidazole tablet. Different dissolution medium was prepared from 200 ml of fluids and 700 ml of 0.1 N HCl. pH was adjusted to pH 1.2. One commonly available marketed brand is used for the study. Dissolution study was performed as per mentioned in Indian pharmacopoeia. For the analysis of study, we used Graph Pad prism version 8 and Microsoft excel. ANOVA followed by Tukey's test was performed for the comparison of dissolution in different fluids
Result: The mean dissolution of metronidazole tablet in Rice water, Lentil soup, Watermelon juice, Mango juice, Pomegranate juice, Black tea and Green tea are significantly different from the dissolution on 0.1 N HCl medium (p<0.05, 95% CI).The dissolution in simulated gastric fluid without enzyme, ORS solution and apple juice are not significantly different from 0.1 N HCl medium (p>0.05, 95% CI).The highest percentage drug release of Metronidazole was found in apple juice (102.78 ± 2.10). In lentil soup dissolution of Metronidazole doesn’t complies with the IP specification (78.69 ± 1.89).
Conclusion: Metronidazole tablet plays a vital role in treatment of diarrhea but before it shows its therapeutic effect on body, it must have to dissolve first. So, dissolution is considered as an important parameter to determine the bioavailability of drug as well as its efficacy. Our study concludes that administration of apple juice can be beneficial on other hand lentil soup can decrease the therapeutic effect of metronidazole tablet.
Keywords
Metronidazole; Dissolution; Different fluids; Lentil soup
Introduction
Metronidazole tablet (400 mg) is included in essential medicine list of Nepal. It is an antiprotozoal anti-parasitic agent used in the treatment of amoebiasis, trichomoniasis, giardiasis, and other parasitic diseases. Since 1960 it has been extensively used as systemic trichomonicide and also shown activity against anaerobic microorganisms including bacteria and protozoa [1]. Metronidazole is used as first line treatment for Clostridium difficile infection which is the leading cause of hospital acquired diarrhoea in developed countries [2]. It is the drug of choice for the treatment of various parasitic infections, Anaerobic infections, Pseudomembranous colitis, Helicobacter pylori and Crohn’s disease [3]. Metronidazole is a prodrug. Unionized metronidazole is selective for anaerobic bacteria due to their ability to intracellularly reduce metronidazole to its active form. This reduced metronidazole then covalently binds to DNA, disrupt its helical structure, inhibiting bacterial nucleic acid synthesis and resulting in bacterial cell death. It is metabolized in liver through oxidation and conjugation process with glucuronic acid. It has 7-8 hours of half-lifeand eliminates through urine [4]. Metronidazole if used during pregnancy may results in high risk of congenital malformations [5]. It shows disulfiram like reactions when administered with alcohol [3].
Metronidazole occurs as pale yellow crystals that is slightly soluble in water and alcohol [6]. According to Indian pharmacopoeia, assay of metronidazole is done by non-aqueous titration method using Perchloric acid as titrant and malachite green as indicator. Methods such as UV spectroscopy and polarography have been reported for the determination of metronidazole tablet [7]. There is need to develop dissolution study that better predict invivo absorption of drug products. This could be achieved if the gastrointestinal tract were successfully reconstructed in vitro [8]. The drug food interaction may affect health status due to altered pharmacokinetics and pharmacodynamics of the drug by increasing drugs bioavailability with the risks of adverse effects and toxicity or decreasing its bioavailability, leading to therapeutic failure [9].
Tablet Dissolution is a standardized method for measuring the rate of drug release from a dosage form. Actually in vitro dissolution of the drug from the tablet matrix relies on many factors, which includes physiochemical properties, nature of formulation and process manufacturing of drugs. Several studies shows that the percentage drug release varies when in vitro studies has been conducted in presence of different juices [3,10,11]. Diarrhea is one of the most common disease in Nepal. During diarrheal condition many fluids has been recommended for replenish of fluid loss and electrolytes and restoring the energy in body. This study deals with the determination of in vitro dissolution release of most commonly available metronidazole tablet considering with different fluids during administration.
Materials and Methods
Reference standard of Metronidazole has been supplied from Lomus Pharmaceuticals Pvt. Ltd. Nepal. All reagents were analytical grade for dissolution studies.
Collection of samples
One commercially available metronidazole film coated tablet was purposively selected from pharmacy located in Kathmandu, Nepal. The sample was within the expiry date. The labeled active ingredients contents of metronidazole were 400 mg and packed in blister packing. The metronidazole tablet was claimed Indian Pharmacopoeia standard. Those samples were properly stored within room temperature before the experiment.
Preparation of standard: 50 mg of metronidazole was weighed and dissolved with 100 ml of 0.1 N HCl. Then, 1 ml of solution was taken and further diluted to 50 ml of 0.1 N HCl.
Preparation of dissolution medium: The pH of all medium were adjusted to 1.2.
Preparation of gastric medium: Preparation of gastric medium (0.1 N HCl, pH 1.2), 50.84 ml of 36.5% w/vHCl was diluted with sufficient water to produce 6000 ml.
Preparation of simulated gastric fluid without enzyme: 2 gm of NaCl was weighed and dissolved in water then 7 ml concentrated HCl was added andthe volume was made 1000 ml with distilled water.
Preparation of oral rehydration salt solution: ORS was randomly purchased from pharmacy and ORS solution was prepared in 1000 ml of water.200 ml of above solution was taken and the volume was made 900 ml with 0.1 N HCl.
Preparation of rice water: 125 ml (1/2 cup) of rice was taken and rinsedthen 2 liter of water was added and the rice was brought to boil on medium heat for five to 10 minutes. The rice wasn’t fully cooked. The rice from the water was separated and cooled. Then 200 ml of previously prepared rice water was diluted to 900 ml with 0.1 N HCl.
Preparation of lentil soup: The Vigna radiate (Mung bean) soup was purchased from hospital canteen. 200 ml of lentil soup and 700 ml of 0.1 N HCl was mixed well before placing in dissolution basket.
Preparation of watermelon juice: The watermelon was sliced and placed in plate with a fork to avoid mess. 500 g of watermelon fresh fruit was taken to which 200 ml of water was added. Then, it was placed in the juicer. The difference between seedless watermelons and watermelons with seeds lies not in their tastes but only in that seedless watermelons contain white edible seeds. After preparation of watermelon juice take 200 ml of water melon solution and 700 ml of 0.1 N HCl solution was mixed well before placing it in dissolution basket [10].
Preparation of juices (apple, mango and pomegranate): The marketed juices were purchased. Each of 200 ml of juices were taken separately and 700 ml of 0.1 N HCl was added in each vessel. The solution was then mixed well and apparatus were assembled. Preparation of tea (black and green): 300 ml water was brought to boil. 2 tea bags were putseparately in a beaker. 200 ml of boiled water was poured into the beaker and infusion was allowed for 2-3 min, after 2-3 min, tea bag were dipped in the beaker for 8-10 times. After preparation of tea 200 ml of each tea solution was taken and 700 ml of 0.1 N HCl solution was mixed well before placing in dissolution basket [11].
Procedure of dissolution method (I.P): Dissolution medium: 900 ml
Apparatus I: 100 rpm
Time: 60 mins
Procedure: Determined the amount of metronidazole dissolved from UV absorbance at wavelength of maximum absorbance at 277 nm of filter portions of solution under test, suitability diluted with 0.1 N HCl, in comparison with a standard solution having a known concentration of IP Metronidazole RS in the same medium.
Tolerance: Not less than 85% of the labeled amount of metronidazole.
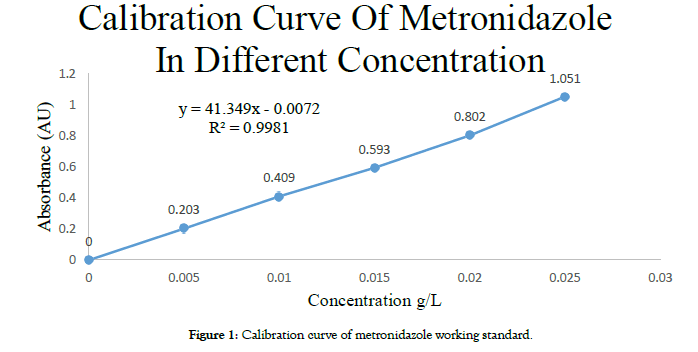
Standard curve preparation: 50 mg of reference metronidazole is dissolved in 0.1 N HCl to make volume 100 ml. From above solution 1, 2, 3, 4, 5 ml is taken respectively and make 100 ml with 0.1 N HCl of each. All gradual concentrations of solution were conducted for plotting standard curve at 277 nm. The corresponding regression data, indicated reasonable linear relationship R2 = 0.9981. The calibration curve was shown in Figure 1.
Figure 1: Calibration curve of metronidazole working standard.
Statistical analysis
For the analysis of this study we used graphpad prism version 8 and Microsoft excel. ANOVA followed by Tukey's multiple comparison test was performed for comparison of% drug release in different dissolution medium with standard dissolution medium.
Results and Discussion
In process quality control parameters
Weight variation test: The objective of weight variation test is to ensure appropriate size of the tablet and the content uniformity of the formulation. The Indian pharmacopoeia provides criteria for tablet weight variation test of intact dosage forms which states that the %weight variation should be within 5% for 400 mg tablet. This brand of tablets compiled within the specifications. The average mean weight of tablet was shown in Table 1.
Table 1: Physical parameters of tablets.
| Weight | Length (mm) | Thickness (mm) | Hardness (Kg/mm2) | Friability % | DT (min) | Assay% | ||
|---|---|---|---|---|---|---|---|---|
| Mean (mg) | %Maximum deviation | %Minimum deviation | ||||||
| 920.17 | 3.03 | 2.61 | 19.28 ± 0.1 | 6.37 ± 0.3 | 5.75 ± 1.3 | 0.021 | 7.45 | 101.37 |
Length and thickness: The tablets of various length and thickness can be prepared without any change of their weights. The length and thickness of tablets depend on the die and punches of the tablet compression machine. As shown as in Table 1 the average length and thickness of tablet was found to be 19.28 mm and 6.37 mm respectively.
Hardness: Hardness influences many tablet properties including disintegration, dissolution, and friability. The average value of hardness was found to be 5.75 (Kg/mm2) which compiled the IP specification which was shown in Table 1.
Friability: Friability test is used to determine physical strength of compressed and uncoated tablets upon exposure to mechanical shock and attrition. The friability of tablet was found to be 0.021% which is within the IP specifications and shown in Table 1.
Disintegration test: Disintegration test is very important for all coated and uncoated tablet because the dissolution rate of drug depends on the disintegration time, which ultimately affect the rate of absorption and subsequent bioavailability of drug. According to IP film coated tablet should disintegrate within 30 mins. Tablets were disintegrate in 7 min and 45 sec which was shown in Table 1. The Metronidazole tablet meets the requirement of IP.
Assay%: Assay is a measure of drug activity expressed in terms of the amount of API (in %) require to produce and effect of given intensity. The assay% of the metronidazole tablet within the range of % of labeled amount of drug and compiled according to IP which was shown in Table 1 average assay of tablet was 101.37%.
Dissolution test
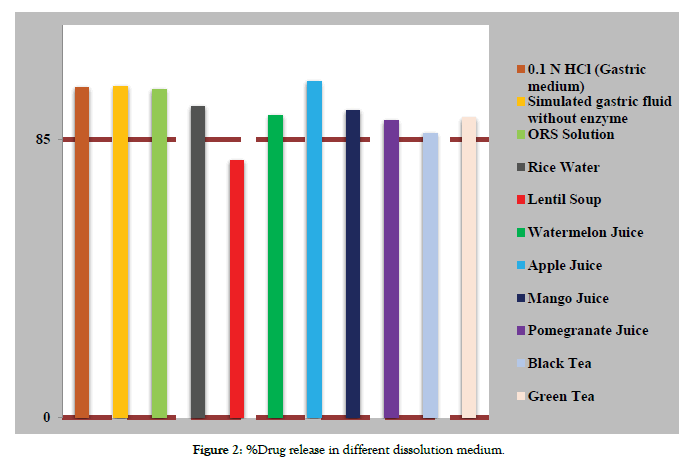
The dissolution of metronidazole tablet was performed in different fluids. The mean %drug release was determined from 6 tablets. According to IP, the limit of %drug release should not be less than 85% of labeled amount in 60 min at 100 rpm. Dissolution percentage of all 6 samples of metronidazole tablets in different fluids were shown in Table 2. The mean %drug release of metronidazole tablet in 0.1 N HCl was found to be 101 ± 2.22 which is within the IP specifications. In simulated gastric fluid without enzyme, the %drug release was found to be 101.28 ± 2.51 which is within the limit range. When dissolution study was performed in ORS solution the %drug release is 100.38 ± 2.52 which passed the test. Dissolution of metronidazole tablet was done in rice water which also pass the specification i.e. 95.16 ± 2.00. Lentil soup was also used as dissolution medium, in this medium the %drug release of metronidazole tablet is decrease to 78.69 ± 1.89. In lentil soup, the dissolution of metronidazole tablet doesn’t compiled with IP specification. The dissolution of Metronidazole was also performed in watermelon juice and %drug release was found to be 92.42 ± 4.72 which passed the limit.The result of the study was also shown in Figure 2. In the study of Amber Nawab et al. the average %drug dissolve in presence of watermelon juice was found to be 97.51 [10] which is slightly higher value than our study.
Table 2: %Drug release of metronidazole samples.
| S.N. | Dissolution Medium | % Drug release | |||||
|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | ||
| 1. | 0.1 N HCl (Gastric medium) | 98.97 | 103.98 | 97.90 | 101.12 | 101.83 | 102.19 |
| 2. | Simulated gastric fluid without enzyme | 98.25 | 104.63 | 103.04 | 99.53 | 99.53 | 102.72 |
| 3. | ORS Solution | 97.97 | 101.29 | 97.97 | 100.39 | 104.62 | 100.39 |
| 4. | Rice Water | 95.79 | 95.50 | 96.37 | 95.50 | 96.66 | 91.18 |
| 5. | Lentil Soup | 77.56 | 76.68 | 76.90 | 79.75 | 81.29 | 79.97 |
| 6. | Watermelon Juice | 85.51 | 91.56 | 88.39 | 95.88 | 96.74 | 96.45 |
| 7. | Apple Juice | 102.94 | 104.70 | 104.00 | 103.51 | 98.74 | 102.83 |
| 8. | Mango Juice | 96.20 | 95.95 | 92.24 | 89.52 | 93.97 | 95.95 |
| 9. | Pomegranate Juice | 86.29 | 92.70 | 88.67 | 95.26 | 91.78 | 91.23 |
| 10. | Black Tea | 86.54 | 87.51 | 86.54 | 86.22 | 86.70 | 87.84 |
| 11. | Green Tea | 91.56 | 91.73 | 92.24 | 93.43 | 90.53 | 90.70 |
Figure 2: %Drug release in different dissolution medium.
Statistical significance was determined between the dissolution on different mediums to investigate the difference between the mean dissolution in all mediums. The ANOVA results indicate that there is significant mean difference between all mediums (p<0.05). The result of ANOVA test is presented in Table 3. Tukey's multiple comparison test is also conducted to compare the differences between the mean dissolution in different medium. The Tukey's test showed that dissolution in Rice water, Lentil soup, Watermelon juice, Mango juice, Pomegranate juice, Black tea and Green tea are significantly different from 0.1 N HCl medium (p<0.05, 95% CI). The dissolution in simulated gastric fluid without enzyme, ORS solution and apple juice are not significantly different from 0.1 N HCl medium (p>0.05, 95% CI). The result of test is presented in Table 4.
Table 3: Drug release in different mediums (ANOVA Table).
| ANOVA Table | SS | Df | MS |
|---|---|---|---|
| Treatment (between columns) | 3142 | 10 | 314.2 |
| Residual (within columns) | 351.3 | 55 | 6.388 |
| Total | 3493 | 65 |
Table 4: Result of Tukey's multiple comparison test. Result is presented only comparison between 0.1 N HCl and other mediums.
| Medium | Mean Diff. | q | Significant? P<0.05 | 95% CI of diff |
|---|---|---|---|---|
| Simulated gastric fluid without enzyme | -0.2850 | 0.2762 | No | -5.191 to 4.621 |
| ORS Solution | 0.5600 | 0.5427 | No | -4.346 to 5.466 |
| Rice Water | 5.832 | 5.652 | Yes | 0.9254 to 10.74 |
| Lentil Soup | 22.31 | 21.62 | Yes | 17.40 to 27.21 |
| Watermelon Juice | 8.577 | 8.312 | Yes | 3.670 to 13.48 |
| Apple Juice | -1.788 | 1.733 | No | -6.695 to 3.118 |
| Mango Juice | 7.027 | 6.810 | Yes | 2.120 to 11.93 |
| Pomegranate Juice | 10.01 | 9.701 | Yes | 5.104 to 14.92 |
| Black Tea | 14.11 | 13.67 | Yes | 9.200 to 19.01 |
| Green Tea | 9.300 | 9.013 | Yes | 4.394 to 14.21 |
Percentage drug release when evaluated in apple juice, the dissolution of metronidazole tablet was within the limit i.e. 102.78 ± 2.10 which is higher %drug release among all other used mediums. in vitro dissolution study of metronidazole tablet was also performed in pomegranate juice and %drug release was found to be 90.98 ± 3.14 which shows significantly decrease the dissolution profile when compared with 0.1 N HCl dissolution medium. Both dissolution medium passed the dissolution test.
For evaluation of metronidazole dissolution profile we also used black and green tea medium. Both medium shows dissolution profile within the limit. The average %drug release was found to be 86.89 ± 0.63 and 91.69 ± 1.06 in black tea and green tea respectively. In black tea, average %drug release was minimum than other used mediums. In both tea mediumdissolution profile significantly decrease when compared with 0.1 N HCl solution. Amber Nawab et al. study shows that 114.66% and 104.12% average drug release in black and green tea respectively [11]. Her study shows higher %drug release in both medium than gastric medium (0.1 NHCl) but our study shows decrease in dissolution profile. The reason behind this may be because of using different brand of tea and different excipients used in different brands.
Conclusion
The present study was performed with a purpose to evaluate dissolution pattern of metronidazole tablet in the presence of different fluids. The drug food interaction can decrease the absorption of drug. Thus, undertaken dissolution testing in dissolution apparatus need to be able to deliver precise and consistent results. The metronidazole tablet which was marketed in Nepal showed higher %drug release in apple juice. In the presence of lentil soup, there was significant decrease in %drug release of metronidazole which doesn’t complies with Indian Pharmacopoeia specification butthe other remaining fluids complied with the IP specification. Conclusively, our study suggests that metronidazole tablet should not be administered with lentil soup.
Data Availability
The data described in this article are new data obtained from this research. The data used to support the findings of this study are included within the article.
Conflict of Interest
The authors declare that they have no conflict of interest to disclose.
Funding
This research received no any funding from any agency, commercial and non-profit sectors. It was a research from department of pharmacy, Manmohan Memorial Institute of Health Sciences, Tribhuvan University, Nepal.
Acknowledgement
Authors would like to thanks Department of pharmacy, Manmohan Memorial Institute of Health sciences, Nepal and also place on record, our sense of gratitude to one and all who, directly or indirectly, have helpedus to complete this research.
REFERENCES
- Tally FP, Sutter VL, Finegold SM. Metronidazole versus anaerobes-in vitro data and initial clinical observations. Calif Med. 1972;117:22.
- Cherian PT, Wu X, Yang L, Scarborough JS, Singh AP, et al. Gastrointestinal localization of metronidazole by a lactobacilli-inspired tetramic acid motif improves treatment outcomes in the hamster model of Clostridium difficile infection. J Antimicrob Chemother. 2015;70:3061-3069.
- Faisal A, Sultana T, Sohel MD, Sumon MH, Kawsar MH et al. In vitro dissolution pattern of metronidazole film coate d tablet in presence of fruit juice. Am J Pharmacol Sci. 2014;2:32-36.
- Noor S, Salam FB, Hima HN, Bhuiyan MS, Chowdhury S. Comparative in-vitro quality evaluation of some brands of metronidazole tablet available in Bangladesh. Int J Appl Res. 2017;3:753-758.
- Caro-Patón T, Carvajal A, Martín de Diego I, Martín-Arias LH, Alvarez Requejo A, et al. Is metronidazole teratogenic? A meta-analysis. Br J Clin Pharmacol. 1997;44:179-182.
- Musa H, Sule YZ, Gwarzo MS. Assessment of physicochemical properties of metronidazole tablets marketed in Zaria, Nigeria. Int J Pharm Pharm Sci. 2011;3:27-29.
- Thulasamma P, Venkateswarlu P. Spectrophotometric method for the determination of metronidazole in pharmaceutical pure and dosage forms. J Chem. 2009;2:865-868.
- Dressman JB, Amidon GL, Reppas C, Shah VP. Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm Res. 1998;15:11-22.
- Santos CA, Boullata JI. An approach to evaluating drug-nutrient interactions. Pharmacother: J Hum Pharmacol Drug Ther. 2005;25:1789-8000.
- Nawab A, Ghayas S, Alam S, Farooq N. Dissolution profile of metronidazole film coated tablet 400 mg (flagyl) in the presence of different juices: An In vitro study. Int Res J Pharm. 2016;7:52-57.
- Nawab A, Alam S, Shoukat N. In-vitro dissolution study of metronidazole film coated tablet 400 mg (flagyl) in the presence of different tea. World J Pharm Sci. 2015:102-115.
Citation: Baniya D, Bohara KB, Pd. Khanal D, Bashyal S, Shahi P (2020) in vitro Dissolution Study of Metronidazole Tablet in Different Fluids. Pharm Anal Acta 12:623. doi: 10.35248/2153-2435.20.11.623.
Copyright: © 2020 Baniya D, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited.