Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2020) Volume 5, Issue 1
Immune Response Towards COVID-19: A Review on Host Body
Nimesh Singh*, Bharat Suthar, Abhay Mehta and Archna PandeyReceived: 24-Apr-2020 Published: 15-May-2020, DOI: 10.35248/2576-389X.5.134
Abstract
As the world is witnessing the epidemic of COVID-19, a disease caused by a novel coronavirus, SARS-CoV-2, emerging genetics and clinical evidences suggest a similar path to those of SARS and MERS. A cascade of viral particles enters the body through the nose, eyes or mouth. Breathing carries some of these particles to the lower respiratory tract where the spike proteins of the coronavirus, acting like a key, lock into epithelial cells that line the respiratory tract as well as those in the the air sacs in the lungs. SARS-CoV-2 is able to stay undetected longer than many flu or coronaviruses and its spike proteins are able to gain entry by unlocking the ACE2 protein on the lung cells. Once in, they hijack the cell’s machinery, replicate and multiply and infect adjoining cells. Like the defining ACE2 proteins on the epithelial cells, viruses too have a tell-tale signature on their surface called antigens and spotting these is what kicks the immune system into action by producing antibodies. Researchers reveal that a broad range of immune cells react to COVID-19 and aid recovery, findings which could inform the development of a potential vaccine.
Keywords
Immunology; Immunotherapy; COVID-19; Corona virus; Vaccine
Introduction
The world experienced the outbreaks of coronavirus infection that threaten global pandemic in 2002-2003 by Severe Acute Respiratory Syndrome (SARS) and in 2011 by Middle East Respiratory Syndrome (MERS). In both cases, the causative agents (SARS-CoV and MERS-CoV, respectively) were newly identified coronavirus in the genus Beta coronavirus with zoonotic origin. At the end of 2019, outbreak of another coronavirus that causes respiratory-related illness was reported in Wuhan, Hubei, China, a disease now officially called “ the Corona Virus Disease 2019; COVID-19”. The coronavirus that is the causative agent of this respiratory disease was identified and its genome is fully sequenced [1].
More than 100 years since the outbreak of the 1918 influenza pandemic, we now seem to face another pandemic. The outbreak of the new coronavirus (SARS-CoV-2) infection is spreading to every continent, forcing us to live with this virus for perhaps a long time. Scientists and clinicians have learned much of coronavirus disease 2019, COVID-19, and its pathogenesis [2]: not all people exposed to SARS-CoV-2 are infected and not all infected patients develop severe respiratory illness. Accordingly, SARS-CoV-2 infection can be roughly divided into three stages: stage I, an asymptomatic incubation period with or without detectable virus; stage II, non-severe symptomatic period with the presence of virus; stage III, severe respiratory symptomatic stage with high viral load [3]. From the point of view of prevention, individuals at stage I, the stealth carriers, are the least manageable because, at least on some occasions, they spread the virus unknowingly: indeed, the first asymptomatic transmission has been reported in Germany [4]. The role of asymptomatic SARS-CoV-2 infected individuals in disseminating the infection remains to be defined.
Two-Phase Immune Responses Induced By Covid-19 Infection
Clinically, the immune responses induced by SARS-CoV-2 infection are two phased. During the incubation and non-severe stages, a specific adaptive immune response is required to eliminate the virus and to preclude disease progression to severe stages. Therefore, strategies to boost immune responses (anti-sera or pegylated IFN α ) at this stage are certainly important. For the development of an endogenous protective immune response at the incubation and non-severe stages, the host should be in good general health and an appropriate genetic background (e.g. HLA) that elicits specific antiviral immunity. Genetic differences are well-known to contribute to individual variations in the immune response to pathogens. However, when a protective immune response is impaired, virus will propagate and massive destruction of the affected tissues will occur, especially in organs that have high ACE2 expression, such as intestine and kidney. The damaged cells induce innate inflammation in the lungs that is largely mediated by proinflammatory macrophages and granulocytes. Lung inflammation is the main cause of life-threatening respiratory disorders at the severe stage [5].
Cytokine Storm and Lung Damage
The cytokine release syndrome (CRS) seems to affect patients with severe conditions. Since lymphocytopenia is often seen in severe COVID-19 patients, the CRS caused by SARS-CoV-2 virus has to be mediated by leukocytes other than T cells, as in patients receiving CAR-T therapy; a high WBC-count is common, suggesting it, in association with lymphocytopenia, as a differential diagnostic criterion for COVID-19. In any case, blocking IL-6 may be effective. Blocking IL-1 and TNF may also benefit patients. Although various clinical sites in China have announced the use of mesenchymal stromal/stem cells (MSCs) in severe cases with COVID-19 infection, solid results have yet to be seen. One caveat is that MSCs need to be activated by IFN γ to exert their anti-inflammatory effects, which may be absent in severely affected patients as T cells are not well activated by SARS-CoV-2 infection. To enhance effectiveness, one could consider employing the “ licensing-approach ” : pretreat MSCs with IFN γ with/without TNF or IL-1 [5].
HLA Haplotypes and SARS-CoV-2 Infection
The major-histocompatibility-complex antigen loci (HLA) are the prototypical candidates for genetic susceptibility to infectious diseases [6,7]. Haplotype HLA-loci variability results from selective pressure during co-evolution with pathogens. Immunologists have found that T-cell antigen receptors, on CD4+ or CD8+ T cells recognize the conformational structure of the antigen-binding-grove together with the associated antigen peptides. Therefore, different HLA haplotypes are associated with distinct disease susceptibilities. The repertoire of the HLA molecules composing a haplotype determines the survival during evolution.
Hyaluronan: A Potential Cause of Fatalities
The innate immune response to tissue damage caused by the virus could lead to acute respiratory distress syndrome (ARDS), in which respiratory failure is characterized by the rapid onset of widespread inflammation in the lungs and subsequent fatality. The symptoms of ARDS patients include short/rapid breathing, and cyanosis. Severe patients admitted to intensive care units often require mechanical ventilators and those unable to breathe have to be connected to extracorporeal membrane oxygenation (ECMO) to support life [8]. CT images revealed that there are characteristic white patches called “ground glass”, containing fluid in the lungs [3]. Recent autopsies have confirmed that the lungs are filled with clear liquid jelly, much resembling the lungs of wet drowning [9] Although the nature of the clear jelly has yet to be determined, hyaluronan (HA) is associated with ARDS [10]; moreover, during SARS infection, the production and regulation of hyaluronan is defective, shown in Figure 1.

Figure 1: After an incubation period, the invading COVID-19 virus causes non-severe symptoms and elicits protective immune responses. The successful elimination of the infection relies on the health status and the HLA haplotype of the infected individual. In this period, strategies to boost immune response can be applied. If the general health status and the HLA haplotype of the infected individual do not eliminate the virus, the patient then enters the severe stage, when strong damaging inflammatory response occurs, especially in the lungs. At this stage, inhibition of hyaluronan synthase and elimination of hyaluronan can be prescribed. Cytokine activated mesenchymal stem cells can be used to block inflammation and promote tissue reparation. Vitamin B3 can be given to patients starting to have lung CT image abnormalities.
Innate Immune Responses to SARS-CoV-2 Infection: Gaining Insight from Strategies Used By SARS-CoV and MERS-CoV
Currently, only limited information is available on the host innate immune status of SARS-CoV-2 infected patients. In one report where 99 cases in Wuhan were investigated, increased total neutrophils (38%), reduced total lymphocytes (35%), increased serum IL-6 (52%) and increased c-reactive protein (84%) were observed [11].
Effective innate immune response against viral infection relies heavily on the interferon (IFN) type I responses and its downstream cascade that culminates in controlling viral replication and induction of effective adaptive immune response. While SARS-CoV and SARS-CoV-2 seem to share the entry receptor of ACE2, MERS-CoV uses dipeptidyl peptidase (DPP)-4 as a specific receptor [12]. The putative receptor of SARS-CoV-2, ACE2, is mainly expressed in a small subset of cells in the lung called type 2 alveolar cells [13]. It has been reported that SARS-Co-V directly infects macrophages and T cells, a key feature in SARS-CoV-mediated pathogenesis [14]. Whether SARS-CoV-2 infects any immune cells are still unknown. Only minimal percentages of monocytes/ macrophages in the lung expressed ACE2.26. If ACE2 is minimally expressed in the potential target immune cells, it is possible that other receptors may exist, or other cellular entry mode is utilized such as antibody-dependent enhancement, shown in Figure 2.
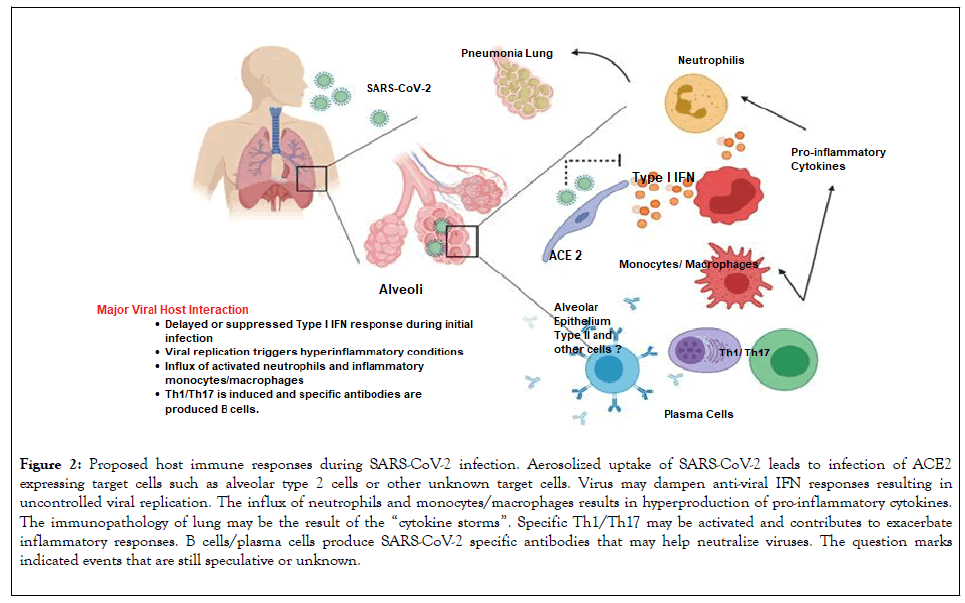
Figure 2: Proposed host immune responses during SARS-CoV-2 infection. Aerosolized uptake of SARS-CoV-2 leads to infection of ACE2 expressing target cells such as alveolar type 2 cells or other unknown target cells. Virus may dampen anti-viral IFN responses resulting in uncontrolled viral replication. The influx of neutrophils and monocytes/macrophages results in hyperproduction of pro-inflammatory cytokines. The immunopathology of lung may be the result of the “cytokine storms”. Specific Th1/Th17 may be activated and contributes to exacerbate inflammatory responses. B cells/plasma cells produce SARS-CoV-2 specific antibodies that may help neutralize viruses. The question marks indicated events that are still speculative or unknown.
Until a vaccine is available, our immune systems will need to adapt unaided to COVID-19.
The immune system is the body’s multi-level defence network against potentially harmful bacteria, viruses and other organisms.
A healthy lifestyle helps one's immune system to be in the best shape possible to tackle pathogens, but it’s better to stop them entering the body in the first place.
The coronavirus pandemic has turned the world’s attention to the immune system, the body’s defence force against diseasecausing bacteria, viruses and other organisms that we touch, ingest and inhale every day.
Think of it as the body ’ s personal army working from the cellular to macro level. Each cell, molecule, tissue and organ in this army plays a vital role in warding off invading pathogens, and also helps guard against internal threats like cancer [15].
The System Has Two Types of Response: Innate and Adaptive
The body’s natural barriers against disease-causing intruders- for example, our skin, the mucous and hairs in our nose, and the acid in our stomachs- are part of our innate immune systems.
Adaptive immunity develops over a lifetime of contact with pathogens and vaccines, preparations which help our immune systems to distinguish friend from foe, shown in Figure 3.
Figure 3: Friend or foe? The adaptive immune system decides.
Vaccination safely teaches our adaptive immune systems to repel a wide range of diseases, and thus protect ourselves and others. There is currently no vaccine for coronavirus, and we may not see one for 18 months or longer. So, for now, our immune systems must adapt unaided to this potentially deadly threat.
Boosting your Immune System against Coronavirus: How to minimize the Risk of Infection?
Improve your diet: The food you eat plays a key aspect in determining your overall health and immunity. Eat low carb diets, as this will help control high blood sugar and pressure. A low carb diet will help slow down diabetes and focus on a protein-rich diet to keep you in good shape. And regularly consume vegetables and fruits rich in Beta carotene, Ascorbic acid & other essential vitamins. Certain foods like mushrooms, tomato, bell pepper and green vegetables like broccoli, spinach are also good options to build resilience in the body against infections.
You can also eat supplements rich in omega 3 & 6 fatty acids for your daily dose, if stepping out to buy groceries is not an option during social distancing. Some natural immunity supplements include ginger, gooseberries (amla) and turmeric. Some of these superfoods are common ingredients in Indian dishes and snacks. There are several herbs that help in boosting immunity like garlic, Basel leaves and Black cumin. Certain seeds and nuts like sunflower seeds, Flax seed, pumpkin seeds and melon seeds are excellent sources of protein and vitamin E.
Don’t compromise on sleep: Good snooze time for 7-8 hours is the best way to help your body build immunity; lesser sleep will leave you tired and impair your brain activity. The lack of sleep will prevent the body from resting and this will impair other bodily functions that will have a direct impact on your immunity. Lack of sleep adversely affects the action of the flu vaccine.
Stay hydrated: Drink up to 8-10 glasses of water every day, to stay hydrated. Hydration will help flush out the toxins from the body and lower the chances of flu. Other alternatives include juices made of citrus fruits and coconut water, to beat the heat.
Don’t skip on exercise: A good diet should be followed by an exercise routine. Remember to exercise regularly; even light exercise will go a long way in releasing the toxins from your body. It is recommended to exercise for 30 to 45 minutes, depending on your stamina. If you have not started exercising yet, then it is a good time to start. There are several Youtube channels and apps to help you exercise at home. Regular exercise improves metabolism, which has a direct correlation with body immunity.
Destress yourself: These are testing times, and a prolonged period of staying indoors has its implications on your mental wellbeing. The growing anxiety around the pandemic is another concern that is affecting millions across the globe. While the uncertainty might be overwhelming, there are few steps we can follow regularly to help relieve our stress, stress is known to have an adverse effect on immunity.
Conclusion
The development of immunity to a pathogen through natural infection is a multi-step process that typically takes place over 1-2 weeks. The body responds to a viral infection immediately with a non-specific innate response in which macrophages, neutrophils, and dendritic cells slow the progress of virus and may even prevent it from causing symptoms. This non-specific response is followed by an adaptive response where the body makes antibodies that specifically bind to the virus. These antibodies are proteins called immunoglobulins. The body also makes T-cells that recognize and eliminate other cells infected with the virus. This is called cellular immunity. This combined adaptive response may clear the virus from the body, and if the response is strong enough, may prevent progression to severe illness or re-infection by the same virus. This process is often measured by the presence of antibodies in blood. WHO continues to review the evidence on antibody responses to SARS-CoV-2 infection.2-17 Most of these studies show that people who have recovered from infection have antibodies to the virus. However, some of these people have very low levels of neutralizing antibodies in their blood, 4 suggesting that cellular immunity may also be critical for recovery. As of 24 April 2020, no study has evaluated whether the presence of antibodies to SARS-CoV-2 confers immunity to subsequent infection by this virus in humans.
Other Considerations
At this point in the pandemic, there is not enough evidence about the effectiveness of antibody-mediated immunity to guarantee the accuracy of an “immunity passport” or “risk-free certificate. ” People who assume that they are immune to a second infection because they have received a positive test result may ignore public health advice. The use of such certificates may therefore increase the risks of continued transmission.
REFERENCES
- Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature [Preprint].2020:19.
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med.2020;382:1708-1720.
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. Jama.2020;323:1061-1069.
- Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med.2020;382:970-971.
- Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol.2014;15:1009-1016.
- Blackwell JM, Jamieson SE, Burgner D. HLA and infectious diseases. Clin Microbiol Rev. 2009;22:370-385.
- Matzaraki V, Kumar V, Wijmenga C, Zhernakova A. The MHC locus and genetic susceptibility to autoimmune and infectious diseases. Genome Biol.2017;18:76.
- MacLaren G, Fisher D, Brodie D. Preparing for the most critically Ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA.2020;323:1245-1246.
- Hallgren R, Samuelsson T, Laurent TC, Modig J. Accumulation of hyaluronan (hyaluronic acid) in the lung in adult respiratory distress syndrome. Am Rev Respir Dis.1989;139:682-687.
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory medicine.2020;8:420-422.
- Y Shi, Y Wang, C Shao, J Huang, J Gan, X Huang, et al. Giuseppe Ippolito & Gerry Melino COVID-19 infection: the perspectives on immune responses, Cell Death & Differentiation.2020;27:1451-1454.
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature [Preprint].2020;15:15.
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med.2020;382:727-733.
- Perlman S, Dandekar AA. Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol.2005;5:917-927.
- Eakachai Prompetchara, Chutitorn Ketloy, Tanapat Palaga, Asian Pac J Allergy Immunol.2020;38:1-9.
Citation: Singh N, Suthar B, Mehta A, Pandey A (2020) Immune Response Towards COVID-19: A Review on Host Body. J Infect Dis Diagn. 5.134. DOI: 10.35248/2576-389X.5.134
Copyright: © 2020 Singh N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.