Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- RefSeek
- Hamdard University
- EBSCO A-Z
- SWB online catalog
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Short Communication - (2020) Volume 9, Issue 2
Formulations 2020: Fast Disintegrating Tablets of Methylene Blue as Diagnostic Aid in Upper GI Endoscopy: Formulation and Evaluation- Sarada Anepu
Sarada Anepu, DOI: 10.35248/2167-1052.20.9.225
Abstract
Barrett’s esophagus is generally evaluated by upper gastro- intestinal endoscopy. In chromoendoscopy technique, methylene blue is used by spraying along the esophagus and upper stomach. The objective of present study is to prepare fast disintegrating tablets of methylene blue, which can disintegrate and dissolve rapidly in the saliva. Fast disintegrating tablets when administered 30 minutes prior to endoscopy will reach the esophagus and provide uniform staining for easy diagnosis during endoscopy. Methods: Fast disintegrating tablets of methylene blue were prepared by varying concentration of superdisintegrant and sublimating agent. Prepared tablets were evaluated after sublimation. Dye-Excipient compatibility studies were performed, and stability studies were carried according to ICH guidelines. Prepared formulations were evaluated in healthy animals. Results: All the precompression blends showed free flowing property and hence, were used in direct compression technique for tablet formulation. Tabletting parameters were within the compendial limits. Disintegration times of all the prepared tablets were less than 60 seconds indicating their suitability as fast disintegrating tablets. In vitro dissolution studies were performed in pH 6.8 phosphate buffer and tablets showing greater than 85% dye release in 10 minutes with minimum friability (10% camphor, 8% crospovidone). Dye- excipient compatibility studies did not show any interactions. Uniform staining along the esophagus indicated the suitability of developed methylene blue tablets. Conclusions: Prepared and evaluated tablets can be administered as diagnostic aid for diagnosis of Barrett’s esophagus, 30 minutes prior to endoscopy.
Introduction
There are many indications for physiological assessment of upper and lower gastrointestinal (GI) tract for which, endoscopes are inserted directly into the organs and tissues are observed in detail. Hence, endoscopy is the most promising and widely used diagnostic tool for GI system. However subtle lesions cannot be identified by normal endoscopy. Chromoendoscopy is a technique in which dye solutions are sprayed using spray catheters. These dyes enhance tissue characterization and easier identification of underlying pathological conditions [1]. However, suction capacity, damage by spray catheter, cost, improper dye distribution etc. stand as major drawbacks associated with spray technique [2,3].
Barrett’s esophagus (BE) is characterized by a replacement of the normal squamous lining of the distal esophagus with metaplastic columnar epithelium. The American College of Gastroenterology defines BE as endoscopically recognizable columnar metaplasia of the esophagus, which is confirmed to have intestinal metaplasia (i.e. goblet cells) in mucosal biopsy specimens [4].
This condition usually results from repeated damage to the esophageal lining, and the most common cause is longstanding gastro esophageal reflux disorder. The intestinal cells have a risk of transforming into cancer cells. As a result, people with BE are advised to have a periodic endoscopy to monitor early warning signs of cancer. Many subjects with BE show no signs or symptoms but it is a known precursor in development of esophageal adenocarcinoma (EAC), a malignancy with a dramatically increasing incidence over the past 50 years and this can be majorly attributed to lifestyle [5].
Methylene blue (MB) is an aniline based dye which was originally produced for fabric industry. It occurs as dark green crystals or crystalline powder, with bronze-like lustre. Solutions in water or alcohol have a deep blue colour. Methylene blue (MB) does not stain normal esophageal mucosa but has a strong affinity for actively absorbing (intestinal type) epithelium such as the normal small intestine mucosa, colon and stomach. Hence, it has been used most commonly to stain specialized intestinal metaplasia within the esophagus, enhancing the detection of Barrett’s esophagus during screening and surveillance [6]. In spray technique, 0.5% w/v methylene blue solution is used for CE [7].
However, there are reports of improper staining with methylene blue during the diagnosis by CE technique [8]. Alternative methods of dye instillation can give better and superior results without economic concern. The aim of the present investigation is to explore the possibility of fast disintegrating methylene blue tablets as an alternative to chromoendoscopic spray technique for accurate diagnosis of BE.
Materials
Methylene blue with CAS No: 122965-43-9, certified by biological Stain Commission was procured from Sigma Aldrich. Camphor was used for pore formation and crospovidone was used as super disintegrant. Saccharin was used as sweetener while Pearlitol SD was used as diluent. All the ingredients used were of analytical grade or pharmaceutical grade.
Methodology
Analytical estimation and pre formulation study
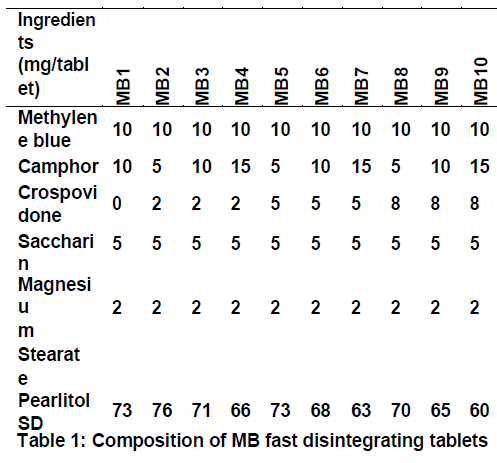
UV-Visible spectrophotometric method was used for estimation of methylene blue [9,10]. All the ingredients were mixed according to formulae shown in Table 1 and evaluated for micromeritic properties.
Formulation of tablets
Powder blend equivalent to a strength of 10 mg of methylene blue was compressed into tablets on a 12-station rotary tablet- punching machine (Karnavati Minipress–II automated compression machine) using 8 mm round plain punches with optimal compression force. Compressed tablets were subjected to the process of sublimation in an oven at 60°C for 6 hours. The compressed tablets were evaluated for general appearance, hardness, thickness, uniformity of weight, friability, disintegration test, content uniformity. The in vitro release studies for all the formulations were performed according to USP type II, paddle method (Labindia dissolution test apparatus). Paddle speed was maintained at 50 rpm and 900 ml of pH 6.8 phosphate buffer was used as the dissolution medium maintained at 37±0.5°C.
Dye-excipient compatibility studies
The incompatibility if any, between the dye and the selected excipients was studied by using FT-IR and DSC.
Surface characteristics
The morphology and surface characteristics of the optimized tablets were studied using scanning electron microscopy (SEM). All the samples were coated with gold–palladium alloy under vacuum. Coated samples were then examined using JSM-6610LV SEM analyzer.
Stability
Stability testing for optimized formulations were performed on a fresh batch of tablets as per the ICH guidelines [11]. Twice the number of tablets required for various tests were packed in high density polyethylene (HDPE) screw capped bottles immediately after initial testing and loaded into the stability chambers Kemi-KHO-3A (Kadavil Electro Mechanical Industries, Kerala, India). The samples were withdrawn at time intervals of 3 and 6 months and evaluated.
In vivo release studies in animal models
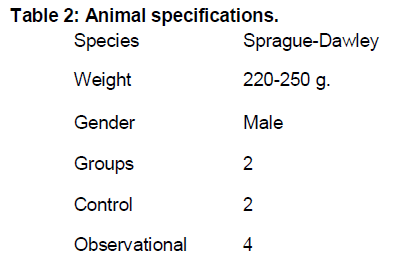
Animal specifications used in present study is shown in Table 2.
After suitable dose conversions, mini tablets were prepared for rats with 0.235 mg. of methylene blue. Tablets of 2 mm diameter. were compressed by using multi pin head punches and evaluated. Six animals were acclimatized to experimental room for 5 days. On the day of experiment, two rats were used as control and remaining 4 rats were used for observation of stain pattern using developed diagnostic aid. All the animals were put on fast overnight, before the day of study. Four rats were stained using methylene blue tablets. During the administration, rat was placed in a body-restraint device, which exposed the animal’s head, and jaws were lifted apart with a wooden tongue depressor. Subsequently, mini tablet was placed in the rat’s mouth, wetting with 2 mL of water. At the same time, a gentle tension was applied to restrain the mouth of the rat for 1 min in order to ensure complete disintegration of the tablet and thus prevented chewing. One animal was euthanized under anesthesia at each time interval i.e. 15, 30, 60 and 120 minutes after dye instillation by sodium pentothal injection and esophagus was isolated and observed. One animal from control group was also subjected to euthanasia and was used to observe esophagus without diagnostic aid. After completion of observational studies, carcass disposal was carried by incineration (Passco Environmental Solutions Pvt. Ltd., Pune).
Results and Discussion
Analytical estimation
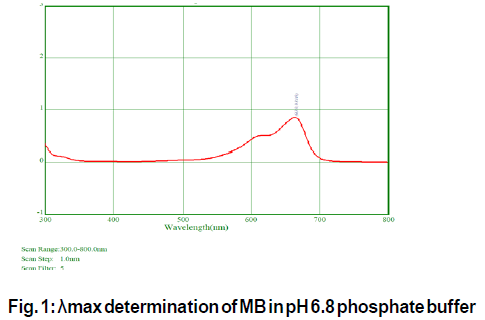
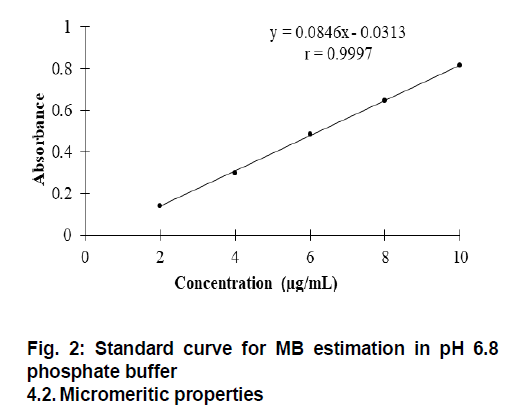
λmax of MB in pH 6.8 phosphate buffer was observed at 663 nm as represented in Fig 1. and was coinciding with the reported value[12]. MB obeyed Beer’s law in the concentration range of 2-10 μg/mL in pH 6.8 phosphate buffer and standard curve is represented in Fig. 2.
Powder blend was evaluated for different flow properties [13]. The results are indicated in Table 3.
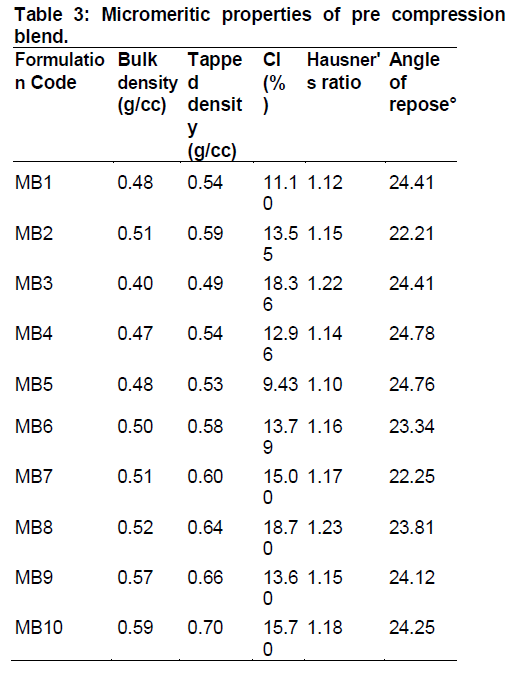
All the prepared blends showed mostly good flow properties which were evident from angle of repose, Hausner’s ratio and CI values and hence, blends were further used for direct compression.
Evaluation of tablets
The sublimation temperature and time were fixed as 60°C for 6 hours respectively after initial trials. Weights of the tablets before and after sublimation are given below in Table. 4 and images of the tablet surface before and after sublimation are given in Fig. 3 and all other tableting characteristics are given in Table 5.
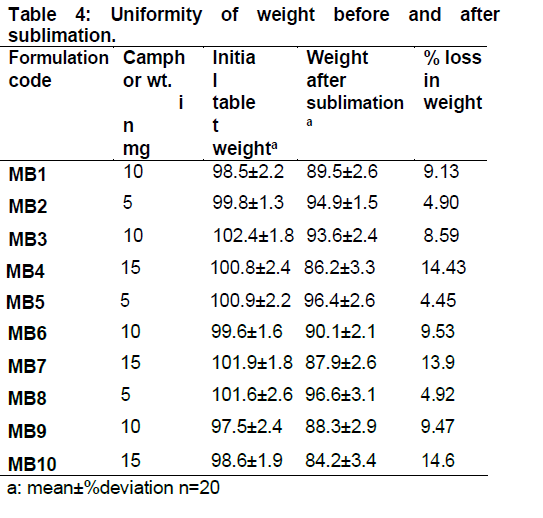

The percentage weight loss in tablet after sublimation was nearly equivalent to the quantity of camphor added, indicating the process of sublimation of camphor. Only traces of camphor were left confirming the suitability of selected temperature and time for complete sublimation of camphor.
The hardness for all the formulations was found to be in the range of 2.1-2.6 kg/cm2. The thickness of tablets was ranged between 2.80 to 2.90 mm. Dye content was acceptable and found to be in the range of 97.3-99.3%. The friability values of all formulations were found to be in between 0.19 to 1.02%. As the concentration of camphor increased, friability increased and all the formulations with 15% camphor showed friability greater than 0.5% (MB4, MB7) while MB10 showed 1.02%. This can be attributed to the increased pores on tablets which might have damaged the tablet integrity. Friability below 1% was an indication of good mechanical resistance of the tablets. However, in the present investigation tablets showing the friability value more than 0.5% were not considered for further studies.
Disintegration time (DT) of the prepared formulations was between 35 seconds to 84 seconds which indicated the suitability for oral fast disintegrating tablets. It was dependent both on the concentration of sublimating agent and concentration of super disintegrant. Thus, all the prepared tablets confirmed to the compendial parameters like, uniformity of weight, friability, content uniformity, hardness, thickness, and disintegration time.
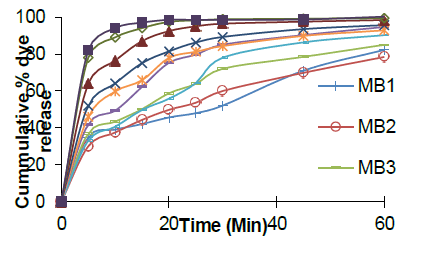
In vitro dissolution study results
In vitro dissolution studies were performed, and results are shown in Fig 4. Amount of sublimating agent and super disintegrant influenced the dye release from all the formulations. All the formulations from MB4 to MB9 showed greater than 90% dye release in studied period. Formulations MB9 and MB10 with 8% concentration of super disintegrant showed about 90% release in 10 minutes while MB8 could release the same in about 20 minutes. MB4, MB7, MB10 with 2, 5 and 8 % of super disintegrant and with 15 % camphor showed more than 40, 50 and 80% dye release in 5 minutes. With increased concentration of super disintegrant, faster dye release was obtained and thus 8% super disintegrant concentration was selected as optimum. The desired dye release of greater than 85 % was achieved by MB9 and MB10 in the stipulated 10 minutes while remaining formulations took longer period for the same. But, MB10 showed friability greater than 1% among all the formulations prepared. MB9, with 10 % camphor and 8% of super disintegrant was selected. More than 85% dye was released in 10 minutes and about 75% was released within 5 minutes indicating its suitability for staining esophagus and upper stomach. Hence, MB9 was selected as optimized formulation and can be administered to the subject 30 minutes prior to endoscopy.
Dye excipient compatibility studies:
Overlay plot of FTIR is given in Fig. 5. and data explained in Table 6 while DSC curves are shown in Fig 6.
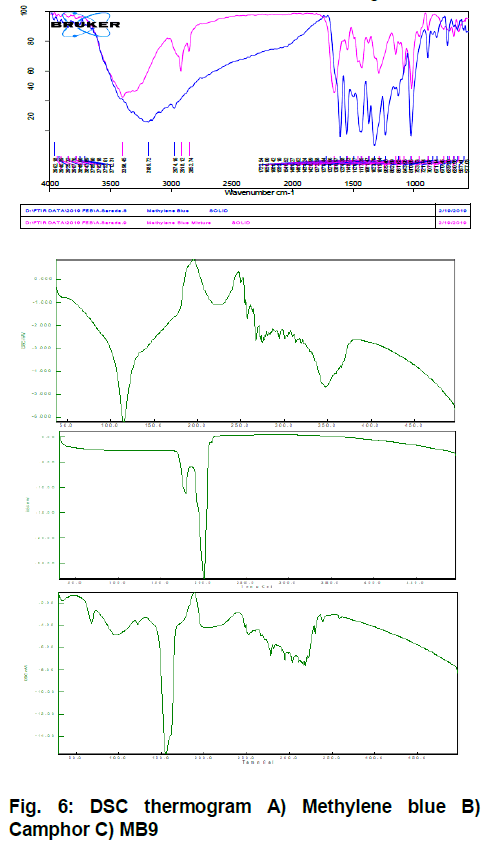
As the optimized formulation clearly showed retention of characteristic bands of the dye in FT-IR, there was no physical or chemical interaction between dye and selected excipient. Endothermic peak as observed with camphor at 200°C is missing in the DSC thermogram of formulation. This can be attributed due to sublimation of camphor. Endothermic peak of methylene blue in formulation showed a slight shift to 140°C from 125°C in pure form.
SEM Analysis:
Surface morphology of the tablets was observed before and after sublimation, SEM images were taken and represented in Fig. 7.

The images clearly indicated the formation of porous surface on the tablet after sublimation. A smooth even surface was observed before sublimation which was replaced by disarrangement and cracks after sublimation as shown.
Stability:
Tablets were evaluated for physical appearance, uniformity of weight, hardness, friability, dye content and in vitro dissolution using similar conditions as described earlier and results for stability studies are represented in Table 7. Dissolution profiles after long term storage conditions and accelerated storage conditions are given in Fig 8 and Fig 9 respectively.
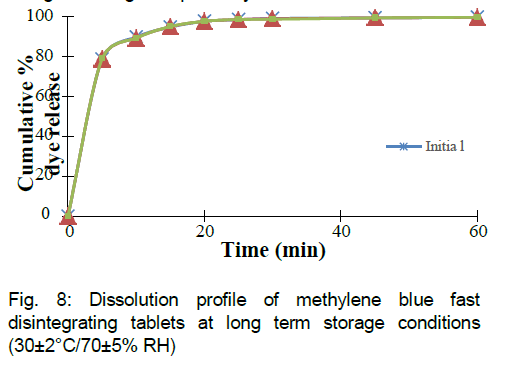
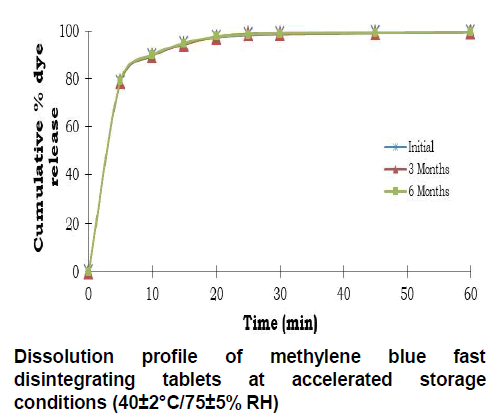
In vitro dissolution profiles were also overlapping and the formulation MB9 was further used for in vivo evaluation
In vivo studies:
Staining of esophagus was observed prominently in the rat sacrificed at 15 minutes time interval. The intensity of the colour decreased at 30 minutes and complete disappearance of the dye was observed at 60 minutes and 90 minutes and the esophagus resembled with that of control group. Esophagus as observed at different time intervals is shown in Fig 8.
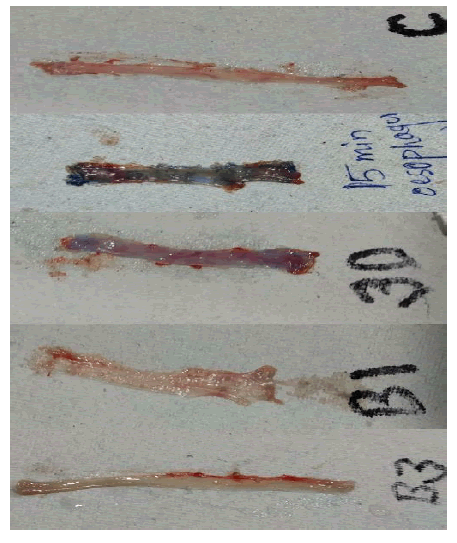
Fig 8: Esophagus at different time intervals
A) Esophagus of control group – colourless B) Esophagus of observational group-15 minutes after dye C) Esophagus of observational group-30 minutes after dye D) Esophagus of observational group-60 minutes after dye E) Esophagus of observational group-90 minutes after dye
In the presence of disease condition, methylene blue is absorbed by the intestinal cells lining up the esophagus. However, as there is no disease condition in these rats, methylene blue release was observed but not persistent. Simulation of Barrett’s esophagus condition in rats involves critical procedures and ethical issues and hence, disease condition was not studied in the present investigation.
Conclusion
The in vivo results clearly indicated uniform staining at esophagus indicating the release of MB from the fast disintegrating tables confirmed the suitability for staining esophagus. Thus, prepared MB9 formulation can be given 30 minutes prior to endoscopy for surveillance and identification of BE condition. Prepared methylene blues tablets can be used as alternative for the spray technique and the dosage form ensures uniform dye distribution without any dye pool up, thus paving way for enhanced diagnostic accuracy.
Ethic clearance: The study was carried out at GLP certified laboratory, (Certificate No: GLP/C-127/2018) Prado, Preclinical Research and Development Organization, Pune, after approval was obtained according to CPCSEA guidelines from Prado Pvt, Ltd. Pune (1723/PO/RcBiBt/S/13/CPCSEA) with approval number Prado/B/1905.
Conflict of interest: The authors certify that they have NO conflict of interest in the subject matter or materials discussed in this manuscript.
Acknowledgements: Corresponding author is thankful to Department of Science &Technology, Government of India for the financial support vide reference No. SR/WOS-A/LS- 430/2016 under Women Scientist Scheme to carry out this work.
REFERENCES
- Trivedi PJ, Braden B. Indications, stains and techniques in chromoendoscopy. QJM: An International Journal of Medicine. 2012; 106(2):117-131.
- Barret M, Camus M, Leblanc S, Coriat R, Prat F, Chaussade S. Toward an easier indigo carmine chromoendoscopy. World Journal of Gastrointestinal Endoscopy. 2015; 7(8):830.
- ASGE Technology Committee, Wong Kee Song LM, Adler DG, Chand B. Chromoendoscopy. Gastrointestinal Endoscopy. 2007; 66:639-649.
- Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. American Journal of Gastroenterology 2008; 103:788–797.
- Daly JM, Karnell LH, Menck HR. National Cancer Data Base report on esophageal carcinoma. Cancer. 1996; 78(8):1820– 1828.
- Canto MI. Chromoendoscopy and magnifying endoscopy for Barrett’s esophagus. Clinical Gastroenterology and Hepatology. 2005;3(7): S12-15.
- https://www.hon.ch/OESO/books/Vol_6_Barrett_s_Esopha gus/Articles/vol1/art028 Accessed on 25.3.2017.
- Kouklakis GS, Kountouras J, Dokas SM, Molyvas EJ, Vourvoulakis GP, Minopoulos GI. Methylene blue chromoendoscopy for the detection of Barrett’s esophagus in a Greek cohort. Endoscopy. 2003; 35(05):383-387.
- O’Neil MJ, Smith A, Heckelman PE, Budavari S. The Merck Index-An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co. Inc. 2006 p4045.
- International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risk of chemicals to man. 2016;108:155-182.
- International Council on Harmonisation. ICH Q1A (R2): Stability testing of new drug substances and products Available at: http://www.ich.org/LOB/media/Q1A R2 Guideline.pdf. Accessed on 16.1.2018.
- Lide DR, editor. Handbook of data on organic compounds. 5. Compounds Pho-Zir. CRC Press; 1994 p4124.
- Carr RL. Evaluating flow properties of solids. Chemical Engineering Journal. 1965; 72(3):163-168.
- Ovchinnikov OV, Evtukhova AV, Kondratenko TS, Smirnov MS, Khokhlov VY, Erina OV. Manifestation of intermolecular interactions in FTIR spectra of methylene blue molecules. Vibrational Spectroscopy. 2016; 86:181-189.