PMC/PubMed Indexed Articles
Indexed In
- Open J Gate
- The Global Impact Factor (GIF)
- Open Archive Initiative
- VieSearch
- International Society of Universal Research in Sciences
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- Publons
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
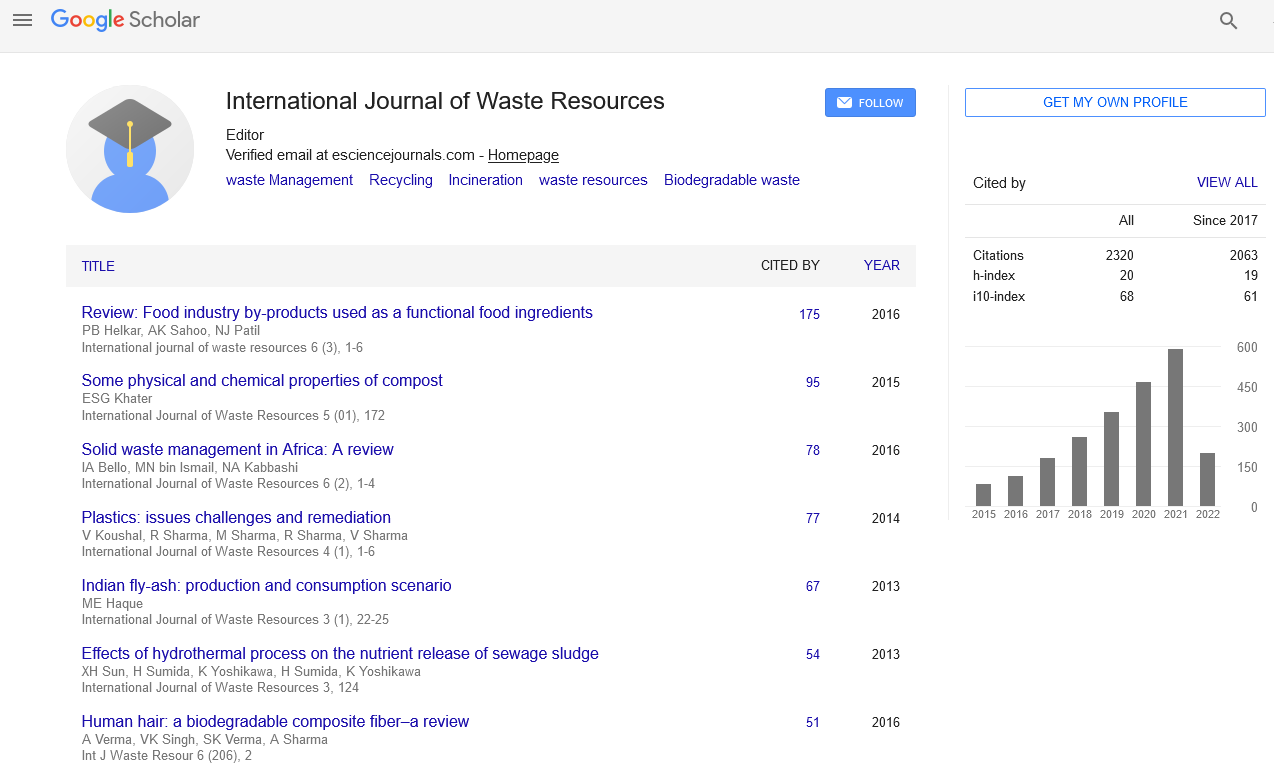
Original Research Article - (2020) Volume 10, Issue 4
Facile Synthesis and Characterization of rGO Decorated NiFe2O4 Nanocomposite Obtained from Waste Ni-Cd/Ni-MH Batteries
Mylarappa M1*, Venkata Lakshmi V2 and Kantharaju S12Research Centre, Department of Chemistry, AMC Engineering College, Bengaluru-560083, Karnataka, India
Received: 25-Jul-2020 Published: 03-Sep-2020, DOI: 10.35248/2252-5211.20.10.385
Abstract
The present study revealed the NiFe2O4/rGO composite synthesized from Ni-Cd/Ni-MH spent by hydrothermal method. The obtained NiFe2O4 nano particles was dispersed effectively on reduced graphene oxide and the obtained composite was subjected to X-Ray powder diffraction (XRD) to know the particle crystallinity, size and structural aspects. The nano sized NiFe2O4 and NiFe2O4/rGO nano composite were exposed to study the surface particle morphology by using Field emission Scanning Electron Microscopy (FESEM). The elements present in the sample was analyzed by using Energy Dispersive X-Ray analysis (EDX), the functional groups identification was done by Fourier Transform Infrared Spectrometer (FTIR) and the thermal stability was studies by using Thermogravimetry analysis.
Keywords
Waste battery; NiFe2O4; NiFe2O4/rGO; Characterization; Thermal analysis
Introduction
Nickel ferrite (NiFe2O4) nanocrystalline is one of the most vital ferrites among alternative ferrites from the spent battery because of most favorable uses in Ferro fluids, gas sensors, storage devices, catalysts and microwave devices [1-7]. Recently, extensive consideration has been paid on NiFe2O4 with variable size, morphology and shape as well analogous applications were studied [8,9]. In the synthesis of NiFe2O4, both chemical and physical methods have been established with different surface morphology. Compared to physical methods, the chemical methods have benefits such as large scale production, low cost and reaction taking place at very low temperature. The nano structured NiFe2O4 has been prepared by different process like sonochemical, polymeric precursor, mechanical alloying, hydrothermal, and co-precipitation methods [10-14].
The literature shows that a few works on the surface morphology controlled preparation of the NiFe2O4 nano particles. Newly, fabricated NiFe2O4 nano sheets using chemical method by Gunjakar et al. [15]. Chu et al. synthesized nano cubes and nanorods of NiFe2O4 through hydrothermal process [16]. Zhang et al. via polyethylene glycol method prepared NiFe2O4 nano particles [17], the hollow sphere NiFe2O4 nano rods and their magnetic properties was studied by Chen L et al. [18]. Also several studies have concentrated on the synthesis of spinel nano ferrites because of their quantum confinement effects, both chemical and physical properties and their surface effects.
The nano NiFe2O4 have AB2O4 structure. In this structure, O specifies the oxygen anion site and A and B shows tetrahedral and octahedral cation sites [19]. The nickel ions (Ni+2) are located in B sites and iron ions (Fe3+) are equally dispersed between A and B sites. It is well known that combined metal oxide nano particles are seemly very attractive to making the electrode materials due to their controlling morphology and size, high surface energy, attractive structural, magnetic and electronic activities, which improve their catalytic performance [20-22].
In the synthesis of nano NiFe2O4, reduced graphene oxide (rGO) was selected as solid subsidiary material to keep the nano NiFe2O4 from aggregation. The rGO based nano composite materials will have increased electrochemical performance like reversibility, capacitive action and cycling stability. The rGO doped nano composite have been widely used as anodes for rechargeable batteries and some recent studies shows the production of rGO based metaloxide anode materials have equitably good development [23,24]. For example, MgFe2O4/rGO composites was displayed excellent cycling stability and rate capability synthesized by Zhang et al. and SnO2/rGO nano hybrid exhibited that electrochemical Na-storage activity enhanced due to amalgamation of reduced graphene oxide [25].
The synthesis and structural aspects of NiFe2O4/rGO nano composite obtained from waste Ni-Cd/Ni-MH batteries by hydrothermal process are not yet described. The current study revealed the facile synthesis of nickel ferrite from waste batteries and the obtained NiFe2O4 was adsorbed on the surface of reduced graphene oxide (rGO) via hydrothermal method.
Experimental Methods
Materials and Methods
The list of chemicals, specifications and suppliers names were employed in this work as indicated in Table 1. The hydrometallurgical method is adapted for the synthesis and structural aspects of rGO doped nickel ferrite nano composite from spent Ni-Cd/Ni-MH batteries.
Table 1: list of chemicals, specifications and suppliers.
| Materials | Specifications | Suppliers |
|---|---|---|
| Waste batteries | AA, and AAA | different manufactures |
| Oxalic acid di-hydrate HO2CCO2H ·2H2O | Molar mass: 192.16 g/mol | Merck, Bangalore, India |
| Citric acid (C6H8O7) | Molecular wt.: 126.07 g/mol | Merck |
| Sulphuric acid (H2SO4) | Molecular wt.: 39.9 g/mol | Merck |
| Sodium Hydroxide (NaOH) | Molecular wt. : 40 g/mol | Merck |
| Reduced graphene oxide (rGO) | Carbon purity :> 99%, Thickness 3-6 nm. | United Nanotech Innovations Pvt. Ltd. Bangalore |
| Ferrous sulphate (FeSO4.7H2O) | Molar mass: 151.9 g/mol | Merck, Bangalore, India |
Synthesis of NiFe2O4 from Waste Batteries
The leached battery powder which obtained from waste Ni-Cd/ Ni-MH batteries taken in a two necked round bottom flask about 1 g and add 100 ml of 3 M sulphuric acid (H2SO4) to dissolve Ni and Cd ions in the powder. When the cations were dissolve with acid, add stoichiometric amount of Mohr’s salt as iron source to solution with constant stirring. The obtained mixture was subjected to analyze the PH of the solution by using sodium hydroxide (NaOH) solution. The reaction mixture was performed at constant agitation speed of 250 rpm and proceeded at 100°C for 60 min, a brown colored precipitate was obtained. The obtained precipitate was dried at 200°C for 24 hours.
Synthesis of NiFe2O4/rGO Nano-Composite
The reduced graphene was added about 1 g in a 250 ml beaker containing 100 ml of distilled water and 1 g of synthesized nano NiFe2O4 obtained from above taken in another beaker separately using ultra-sonication for better dispersion for 30 min. The well dispersed NiFe2O4 nano particles was decanted into the solution containing rGO in water with constant stirring. The entire mixture was maintained constant speed at 100°C for one hour. The obtained NiFe2O4/rGO nano composite was filtered, dried and washed. The precipitate was kept in oven for 24 hours at 100°C. The Flow chart and illustration of synthesis of NiFe2O4/rGO Nano composite as shown below Figure 1.
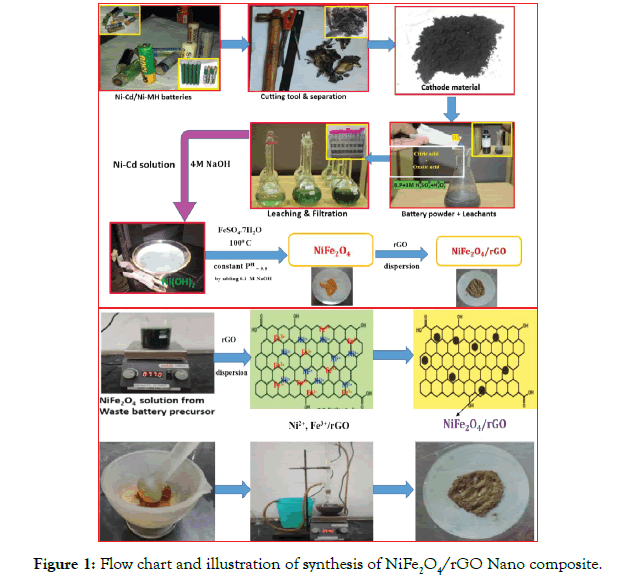
Figure 1: Flow chart and illustration of synthesis of NiFe2O4/rGO Nano composite.
Results and Discussions
X-Ray Diffraction Studies
X-Ray diffraction (XRD) spectra of NiFe2O4 and NiFe2O4/rGO nano composite are shown in Figure 2. In Figure 2a, the measured average crystallite size of NiFe2O4 nanoparticle was about 9 nm and the graphic representation of a NiFe2O4 unit cell is presented as an introduced image in Figure 2a. The diffraction peaks of NiFe2O4 which indicated the formation of crystalline phase of NiFe2O4 and broadening of the peaks were confirmed the nano crystalline nature of the powder. In Figure2b, the particle size of NiFe2O4 nano particle and NiFe2O4/rGO nanocomposites was found to be 9 nm and 13.4 nm respectively by using the Scherrer’s equation. In NiFe2O4/rGO nanocomposites the peak intensity (220) was disappear completely recommending that reducing crystal size of NiFe2O4, which reveals the growth of NiFe2O4 particles were essentially restricted after the fusing of rGO [26]. The extra peaks at about 27°, 29° and 46° are observed in Figure 2a were due to the contamination of the sample or that my occur during the leaching process of the waste batteries.
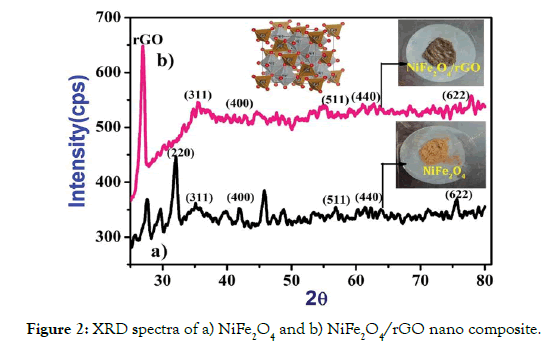
Figure 2: XRD spectra of a) NiFe2O4 and b) NiFe2O4/rGO nano composite.
We observed that, the peak intensities of NiFe2O4/rGO nanocomposites compared to NiFe2O4 decreases with increase in average particle size and doped rGO will decreased intensity peak, which shows the growth of NiFe2O4 was controlled after the doping of rGO dispersion. The Williamson and Hall method and Scherrer’s equation was employed to calculate the particle sizes of NiFe2O4 and NiFe2O4/rGO nano composite. According to Williamson and Hall, when lattice micro stain and domain were all together concurrently working their mutual effects provides the final line broadening of full width half maximum (FWHM) in which the sum of lattice distortion and particle grain size. The following equation gives a straight line between β cosθ and 4sinθ where ε is associated strain with nano composite.
 (1)
(1)
From the above equation, the average crystalline size (D) is measured by the intercept 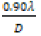 and strain (ε) was calculated by the slope of the line obtained from the graph. The results obtained from the equation were analogous to the particle size calculated from the Scherrer’s equation. Particle size and other structural parameters of NiFe2O4 and NiFe2O4/rGO as indicated in Table 2. The density and stacking fault were calculated by the following equations.
and strain (ε) was calculated by the slope of the line obtained from the graph. The results obtained from the equation were analogous to the particle size calculated from the Scherrer’s equation. Particle size and other structural parameters of NiFe2O4 and NiFe2O4/rGO as indicated in Table 2. The density and stacking fault were calculated by the following equations.
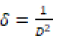
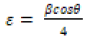
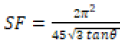
Table 2: Particle size and other structural parameters of NiFe2O4 and NiFe2O4/rGO.
| Name of the sample | FWHM (rad) | Crystalline Size(nm) | Strain (ε)×10-3 | stacking fault SF | dislocation density (δ) (105 lin m-2) |
|---|---|---|---|---|---|
| NiFe2O4 | 1.28 | 9 | 0.27 | 0.321 | 0.0123 |
| NiFe2O4/rGO | 0.632 | 13.4 | 0.140 | 0.353 | 0.0055 |
Field Emission Scanning Electron Microscopy
The field emission scanning electron microscopy (FESEM) was used to analyze the surface morphology images of NiFe2O4 and NiFe2O4/ rGO nano composite as shown in the Figures 3a-3d. The surface morphology of particles are composed of various quadrangular shape with 1μm and 2 μm as indicated in Figures 3a and 3b. In Figures 3c and 3d, clearly shows that when reduced graphene was dispersed in the ferrite, The NiFe2O4 can be spread on the surface of nano ferrite forming NiFe2O4/rGO nano composite and the morphology of the particles are well distributed and some have little aggloromation as shown in the Figures 3c and 3d.
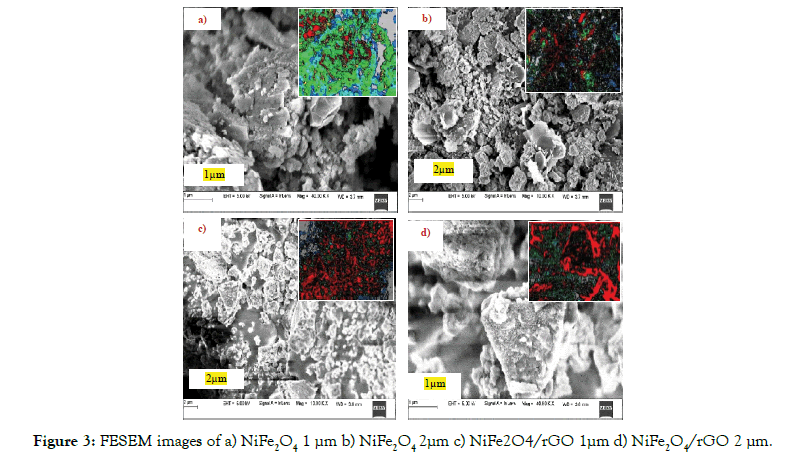
Figure 3: FESEM images of a) NiFe2O4 1 μm b) NiFe2O4 2μm c) NiFe2O4/rGO 1μm d) NiFe2O4/rGO 2 μm.
EDAX Analysis
From elemental analysis data, observed that C, Ni, Fe and O peaks, which confirms the purity of our synthesized sample. The chemical composition of NiFe2O4 and rGO/NiFe2O4 nano composite was indicated in Table 2. The qualitative analysis of C, Ni, Fe and O in the NiFe2O4 and NiFe2O4/rGO nanocomposites are determined by using EDX analysis as shown in Figures 4a and 4b. The elemental composition of NiFe2O4 is composed of 20.45% oxygen, 7.70% carbon, 2.27% Nickel and 21.69% iron respectively. Similarly in Figure 4b, the chemical composition of NiFe2O4/rGO is consist of 26.0 % oxygen, 10.0% carbon, 2.59% Nickel and 21.69% iron respectively.
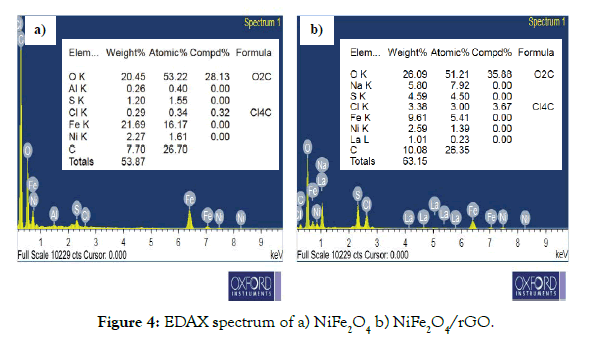
Figure 4: EDAX spectrum of a) NiFe2O4 b) NiFe2O4/rGO.
Fourier Transforms Infra-Red Spectrometer Studies
The functional groups attached for NiFe2O4 and NiFe2O4/rGO was performed by using Fourier transforms infra-red spectrometer (FT-IR). FTIR spectrum of NiFe2O4 NiFe2O4/rGO nano composite as shown in Figures 5a and 5b respectively.
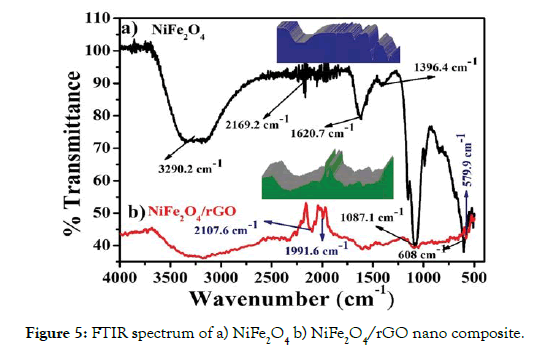
Figure 5: FTIR spectrum of a) NiFe2O4 b) NiFe2O4/rGO nano composite.
In Figure 5a, the absorption frequency at 3290.2 cm-1 and 2169.2 cm-1 could be attributed to the symmetric vibration of -OH groups. The bands with peaks detected at 1087 cm-1 was assigned to O-H bending vibration. The absorption peaks near 162°Cm-1 and 608 cm-1 appeared in NiFe2O4 which are attributed to the skeletal vibration of metal-oxygen bonds of the NiFe2O4 particles. The vibration bands of Fe and Ni are found in the range of 1000–120°Cm-1 is the region of the vibrational bands of Fe2O3 in octahedral site and Ni–O in tetrahedral sites present in the samples [27-29]. In Figure 5b, the bands at about 21076 cm-1 and 1991.6 cm-1 are represented the stretching vibration of C=O and The bending vibration of H-O-H was assigned at 2107.6 cm-1. The C-O bending vibration occur at absorbed frequency 108 7.1 cm-1 and peak 1622 cm-1 demonstrate the C=C stretching vibration of reduced graphene oxide sheets. The M-O bond absorbed at 579.9 cm-1 [30-32] and the rGO absorption bands resemble to the oxygencontaining functional groups are vanished in rGO doped nickel ferrite nano composite and the oxygen-containing functional groups almost vanished in NiFe2O4/rGO nano composite shows that oxygen-containing functional groups were almost removed in rGO dispersion reaction [33-35].
Thermogravimetry Analysis
The TGA of NiFe2O4 and NiFe2O4/rGO nano composite were studied by TGA and the results were shown in Figures 6a and 6b.
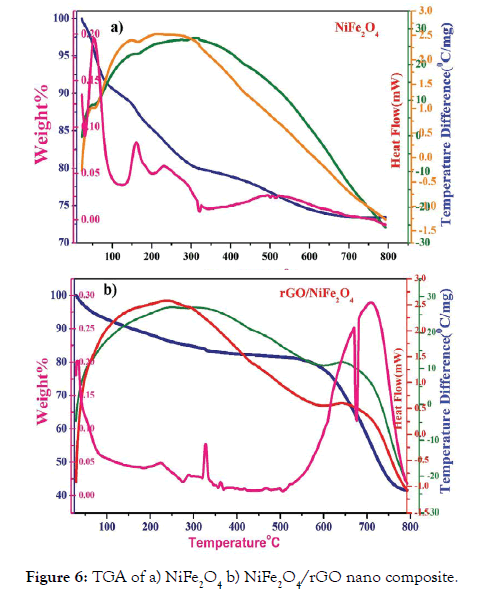
Figure 6: TGA of a) NiFe2O4 b) NiFe2O4/rGO nano composite.
In Figure 6a, The NiFe2O4 and NiFe2O4/rGO nano particles were The NiFe2O4 and NiFe2O4/rGO nano particles were prepared from spent battery as appeared in Figures 6a and 6b. In Figure 6a uncovers the NiFe2O4 nano particles has three separate warm disintegration steps. The main weight reduction stage in the temperature scope of 25°C-100°C, which was joined by an extremely little endothermic pinnacle is because of the loss of water particles in the NiFe2O4. The second region begins ~ 145°C and ends at 324°C with a weight loss of 80% which might be due to the initial breakdown of reactants. Almost no weight loss over ~708°C indicates the completion of thermal decomposition and formation of spinel ferrite [36].
In Figure 6b the rGO decorated nickel ferrite nano composite indicates three weight reduction forms. The weight loss below 100°C was removal of moisture in the sample. The weight reduction take place from 100 to 326°C, which caused by the destruction of oxygenated functional groups. Next a massive weight reduction happens between 326°C and 586°C. It tends to be dispersed to the ignition and disintegration of carbon molecule. The final exothermic phase was around 586°C and 796°C can be recognized for the rGO decorated NiFe2O4 composite and the weight loss occurred between 586°C and 796°C was indicating the conversion of reactants into the final products [37].
Conclusion
From this studies average particle sizes of NiFe2O4 and NiFe2O4/ rGO composites of particle average size ranging from 9 to 13.4 nm were synthesized successfully from waste battery collection through hydrothermal method. The surface morphology of NiFe2O4 and NiFe2O4/rGO having quadrangular shape with NiFe2O4 nano particles are well covered on the rGO surface. The elements C, O, Ni and Fe were the main chemical constituents present in the sample which was confirmed by EDX analysis. From infrared analysis, the high frequency band at 579 and 608 cm-1 was corresponds to M-O bonds in NiFe2O4 and NiFe2O4/rGO nano composite. From Thermal studies, the sample NiFe2O4 nano particles can withstand the temperature up to 706°C and NiFe2O4/rGO nano composite up to 796.6°C respectively.
REFERENCES
- Sugimoto M. The past, present, and future of ferrites. Journal of the American Ceramic Society. 1999;82(2):269-80.
- Reddy CG, Manorama SV, Rao VJ. Semiconducting gas sensor for chlorine based on inverse spinel nickel ferrite. Sensors and Actuators B: Chemical. 1999;55(1):90-5.
- Reddy CG, Manorama SV, Rao VJ. Preparation and characterization of ferrites as gas sensor materials. Journal of materials science letters. 2000;19(9):775-8.
- Dube G, Darshane V. J, (1993) Mol Catal. 79:285-96.
- Sousa MH, Hasmonay E, Depeyrot J, Tourinho FA, Bacri JC, Dubois E, et al. NiFe2O4 nanoparticles in ferrofluids: evidence of spin disorder in the surface layer. Journal of magnetism and magnetic materials. 2002;242:572-4.
- Ramankutty CG, Sugunan S. Surface properties and catalytic activity of ferrospinels of nickel, cobalt and copper, prepared by soft chemical methods. Applied Catalysis A: General. 2001;218(1-2):39-51.
- Šepelák V, Baabe D, Mienert D, Schultze D, Krumeich F, Litterst FJ, et al. Evolution of structure and magnetic properties with annealing temperature in nanoscale high-energy-milled nickel ferrite. Journal of Magnetism and Magnetic Materials. 2003 Feb 1;257(2-3):377-86.
- Gunjakar JL, More AM, Gurav KV, Lokhande CD. Chemical synthesis of spinel nickel ferrite (NiFe2O4) nano-sheets. Applied Surface Science. 2008;254(18):5844-8.
- Wang H, Zhang W, Zhang F, Cao Y, Su W. The effect of 3-aminopropyltrimethoxysilane on the formation of NiFe2O4/SiO2 nanocomposites. Journal of magnetism and magnetic materials. 2008;320(13):1916-20.
- Yang JM, Tsuo WJ, Yen FS. Preparation of ultrafine nickel ferrite powders using mixed Ni and Fe tartrates. Journal of Solid State Chemistry. 1999;145(1):50-7.
- Prasad S, Gajbhiye NS. Magnetic studies of nanosized nickel ferrite particles synthesized by the citrate precursor technique. Journal of Alloys and Compounds. 1998;265(1-2):87-92.
- Chen DH, He XR. Synthesis of nickel ferrite nanoparticles by sol-gel method. Materials Research Bulletin. 2001 May 1;36(7-8):1369-77.
- Liu C, Zou B, Rondinone AJ, Zhang ZJ. Chemical control of superparamagnetic properties of magnesium and cobalt spinel ferrite nanoparticles through atomic level magnetic couplings. Journal of the American Chemical Society. 2000 Jul 5;122(26):6263-7.
- Zhou J, Ma J, Sun C, Xie L, Zhao Z, Tian H, et al. Low‐Temperature Synthesis of NiFe2O4 by a Hydrothermal Method. Journal of the American Ceramic Society. 2005;88(12):3535-7.
- Gunjakar JL, More AM, Gurav KV, Lokhande CD. Chemical synthesis of spinel nickel ferrite (NiFe2O4) nano-sheets. Applied Surface Science. 2008;254(18):5844-8.
- Chu XF, Jiang DL, Zheng CM. The preparation and gas-sensing properties of NiFe2O4 nanocubes and nanorods. Sens. Actuators B. 2007;123(2):793-7.
- Zhang DE, Zhang XJ, Ni XM, Zheng HG, Yang DD. Synthesis and characterization of NiFe2O4 magnetic nanorods via a PEG-assisted route. Journal of Magnetism and Magnetic Materials. 2005;292:79-82.
- Chen L, Dai H, Shen Y, Bai J. Size-controlled synthesis and magnetic properties of NiFe2O4 hollow nanospheres via a gel-assistant hydrothermal route. Journal of Alloys and Compounds. 2010;491(1-2):L33-8.
- O'handley RC. Modern magnetic materials: principles and applications. Wiley; 2000.
- Srivastava M, Singh J, Kuila T, Layek RK, Kim NH, Lee JH. Recent advances in graphene and its metal-oxide hybrid nanostructures for lithium-ion batteries. Nanoscale 7: 4820-68
- Son DI, Yang HY, Kim TW, Park WI. Transparent and flexible ultraviolet photodetectors based on colloidal ZnO quantum dot/graphene nanocomposites formed on poly (ethylene terephthalate) substrates. Composites Part B: Engineering. 2015;69:154-8.
- Tang Q, Chen M, Wang L, Wang G. A novel asymmetric supercapacitors based on binder-free carbon fiber paper@ nickel cobaltite nanowires and graphene foam electrodes. Journal of Power Sources. 2015;273:654-62.
- Qin W, Li D, Zhang X, Yan D, Hu B, Pan L. ZnS nanoparticles embedded in reduced graphene oxide as high performance anode material of sodium-ion batteries. Electrochimica Acta. 2016;191:435-43.
- Walter M, Zünd T, Kovalenko MV. Pyrite (FeS 2) nanocrystals as inexpensive high-performance lithium-ion cathode and sodium-ion anode materials. Nanoscale. 2015;7(20):9158-63.
- Zhang Y, Xie J, Zhang S, Zhu P, Cao G, Zhao X. Ultrafine tin oxide on reduced graphene oxide as high-performance anode for sodium-ion batteries. Electrochimica Acta. 2015;151:8-15.
- Shetty K, Lokesh SV, Rangappa D, Nagaswarupa HP, Nagabhushana H, Anantharaju KS, et al. Designing MgFe2O4 decorated on green mediated reduced graphene oxide sheets showing photocatalytic performance and luminescence property. Physica B: Condensed Matter. 2017;507:67-75.
- Ren L, Qiu J, Wang S. Photovoltaic properties of graphene nanodisk-integrated polymer composites. Composites Part B: Engineering. 2013;55:548-57.
- Guo L, Shen X, Meng X, Feng Y. Effect of Sm3+ ions doping on structure and magnetic properties of nanocrystalline NiFe2O4 fibers. Journal of Alloys and Compounds. 2010;490(1-2):301-6.
- Tahir MB, Iqbal T, Hassan A, Ghazal S. Wet chemical co-precipitation synthesis of nickel ferrite nanoparticles and their characterization. Journal of Inorganic and Organometallic Polymers and Materials. 2017;27(5):1430-8.
- Li Y, Zhang Z, Pei L, Li X, Fan T, Ji J, et al. Multifunctional photocatalytic performances of recyclable Pd-NiFe2O4/reduced graphene oxide nanocomposites via different co-catalyst strategy. Applied Catalysis B: Environmental. 2016;190:1-1.
- Qiu Q, Chen Y, Xue J, Zhu J, Fu Y, He G, et al. One-step solvothermal synthesis of spherical spinel type NiFe2− xMnxO4-RGO as high-performance supercapacitor electrodes. Ceramics International. 2017 43(2):2226-32.
- Qi F, Xu B, Chu W. Heterogeneous catalytic ozonation of phenacetin in water using magnetic spinel ferrite as catalyst: Comparison of surface property and efficiency. Journal of Molecular Catalysis A: Chemical. 2015;396:164-73.
- Zhao Y, He G, Dai W, Chen H. High catalytic activity in the phenol hydroxylation of magnetically separable CuFe2O4–reduced graphene oxide. Industrial & Engineering Chemistry Research. 2014;53(32):12566-74.
- Cong HP, He JJ, Lu Y, Yu SH. Water‐soluble magnetic‐functionalized reduced graphene oxide sheets: in situ synthesis and magnetic resonance imaging applications. Small. 2010;6(2):169-73.
- Fu M, Jiao Q, Zhao Y. Preparation of NiFe2O4 nanorod–graphene composites via an ionic liquid assisted one-step hydrothermal approach and their microwave absorbing properties. Journal of Materials Chemistry A. 2013;1(18):5577-86.
- Baythoun MS, Sale FR. Production of strontium-substituted lanthanum manganite perovskite powder by the amorphous citrate process. Journal of Materials Science. 1982;17(9):2757-69.
- Uddin ME, Kim NH, Kuila T, Lee SH, Hui D, Lee JH. Preparation of reduced graphene oxide-NiFe2O4 nanocomposites for the electrocatalytic oxidation of hydrazine. Composites Part B: Engineering. 2015;79:649-59.
Citation: Mylarappa M, Venkata Lakshmi V, Kantharaju S (2020) Facile Synthesis and Characterization of rGO Decorated NiFe2O4 Nanocomposite Obtained from Waste Ni-Cd/Ni-MH Batteries, Int J Waste Resour 10: 385. doi: 10.35248/2252-5211.20.10.385
Copyright: © 2020 Mylarappa M, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.