Indexed In
- Open J Gate
- Genamics JournalSeek
- Ulrich's Periodicals Directory
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Scholarsteer
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2019) Volume 9, Issue 1
Extraction and Facilitated Transport of Cd(II) Ions across Triethylenediamine-Carbon Tetrachloride Supported Liquid Membranes
Naheed Bibi1*, Hanif Ur Rehman2, Kamin Khan2, Aisha Kanwal1, Rana Gul1, Khurshid Ali2 and Nauman Ali12Department of Inorganic Chemistry, Sarhad University of Science and Information Technology, Peshawar, Pakistan
Received: 24-Jan-2019 Published: 07-Mar-2019
Abstract
The extraction of Cadmium (II) (Cd(II)) ions across a Supported Liquid Membrane (SLM) using Triethyleneamine (TEDA) in Carbon tetrachloride (CCl4) as a carrier, was investigated. The aforementioned carrier was embodied within the microporous polypropylene membrane. Hereby, we present the results of optimization of certain conditions like acid and carrier concentration in feed and membrane phase respectively, stripping phase makeup, and their effects on stripping of the metal ions from the feed solution. 1.0 M HNO3 in the feed solution, 1.5 M in stripping phase, 3.75 M of TEDA in membrane phase and 2.36 × 10-3 mol/dm3 of Cd(II) metals ions concentrations were found to be the optimum conditions for the extraction of Cd(II). Some theoretical equations were proposed which were beneficial in investigating the extraction mechanism and stoichiometry of the physio-chemical processes occurring in the organic membrane phase. Characterization of the SLM through flux, permeability and diffusion coefficient and an investigation into its stability for applications in the industry were also carried out.
Introduction
Various classical techniques are commonly employed, for the extraction and separation of Cd ions from certain solutions. Ion exchange, solvent extraction, carbon adsorption, adsorption chromatography, separation by Carbon Nanotubes (CNTs), filtration, distillation, evaporation, preparative chromatography, usage of particular plants and micro-organisms are just a few to be named [1-4]. However, these extraction methods are deemed unsuitable for large scale stripping of Cd due to the use of a voluminous amount of solvents, problematic and unsafe disposal of large amounts of toxic sludge, inappropriate handling and operation difficulties [5,6].
Membrane technology is playing a key role in safe extractions and holds importance in its vast range of applications. A vital aspect of the selection of a membrane is the transit rate of various chemical species across the membrane. In controlled drug delivery, the transit or permeation rate is intermediated from the source to the body or point of action whereas, in separation techniques where membranes are employed, its main purpose involves the permeation of certain component of the mixture while blocking others [7].
Cd(II) has been extracted across SLM which was incorporated with thiacalix [4] arene as an extractant. The extractant exhibits a higher flux of Cd(II) as compared to that of lipophilic extractant lasalocid [8].
In a comparative transport study, the extraction of Cd(II) through SLM impregnated with tri-n-octylamine in kerosene has been compared with that of dispersion hybrid liquid membrane. It has been reported that the dispersion hybrid liquid membrane is highly efficient than SLM because of high percentage transport of solute in the strip, permeability coefficient, depletion of extractant, extraction efficiency and transport flux [9].
Danesi, et al. [10] reported the stripping of Zn(II) and Cd(II) from a chloride solution of the feed into the ammonium acetate solution in the strip through supported liquid membrane impregnated with trilaurylammonium chloride solution in triethyl benzene as an extractant.
The transport of Cadmium (Cd), Copper (Cu), and Lead (Pb) ions were investigated through a hollow fiber SLM consisting of 1,10-didecyldiaza 18-crown-6 and fatty acid, where a 1:1 combination of toluene and phenyl hexane was used as a mobile carrier [2]. Extraction flow and non-flow system of a single hollow fiber module were investigated for metal ions. For examining flow system for a specific recipient solution, it was retained in the cavities of the membrane to investigate the consequences and outcome of various elements, the volume of the samples, the concentration of metal ions and the flow rates of the solution in relation to the preconcentration factor [11,12].
Similarly, the extraction and permeation study of Cd(II) ions with various carriers like kelex 100, LIX 54 and aliquat 336 soaked in an SLM was also studied [13]. The flux for LIX-54 was 7.9 × 1011, for kelex 100 was 1.5 × 10-10 and for aliquat was 1.12 × 10-9 mol/cm2 s.
Daoud, et al. [14] worked on the extraction of Cd(II) using a SLM with Cyanex-302 in kerosene in the presence of other metal ions like Cu(II) and Zn(II) and found that the transport of Cd(II) increased with an increase in stirring rate, and also feed and strippant concentration. Interestingly, complete removal of Cd(II) was achieved at pH 2 using Cyanex-302 [14]. Another extraction process for the removal of Cd(II) from neutral and acidic media was developed using Cyanex 923 where extraction and rate of transport was found to be governed by H+ and Clions. The transport of the ions dropped down due to the appearance of another phase in the pores of the flat sheet supported liquid membrane, however, this condition could be prevented by either using diluent like solvesso 200 or keeping the Cd(II) concentration low. On the other hand, in neutral chloride media, the extraction seemed to be indifferent to the Cd(II) ions concentration and pore size of the supporting membrane. In hollow fiber strip dispersion system, the transport of Cd(II) was efficient despite the formation of another phase in acidic chloride medium [15].
Cd(II) ions transport through SLM containing TBP/cyclohexane from high salinity chloride solution to an EDTA solution was studied by Nowier, et al. [16] It was shown that permeability increased with increased pH, increased tri-n-butyl phosphine (TBP) in the membrane and strip concentration. The extraction rate dropped to a low value after a further increase in TBP concentration. The process was controlled by chemical reactions. Optimum extraction occurred at 25°C with a 50% TBP and 0.04M EDTA in the strip.
Altin, et al. [17] investigated the optimum conditions for extraction of Cd(II) using EDTA (0.06M) as a stripping solution with aliquat 336 as a carrier, from a feed solution of 2M HCl, mainly because EDTA has a high capacity of complexation with Cd(II) ions. The flow rates were found to be 50 ml/min and 80 ml/min for 2M HCl and EDTA solution, respectively, with efficiency above 80%.
He, et al. [18] compared the rate of extraction of Cd(II) ions using two different carrier solutions i.e., tri-n-octylamine (TNOA) and tricapryl amine (N235) in CCl4 solution with ammonium acetate solution as a strip. The optimum conditions were investigated extensively and it was found the transport was much efficient with TNOA. The extraction activation energies were calculated to be 6.8 and 4.8 kJ/mol for tricapryc amine and tri-n-octylamine, respectively.
The present work is aimed to extract Cd(II) ions from spent cadmium-nickel battery, to optimize the conditions for maximum extraction, and investigate the physical and chemical processes affecting the efficiency of supported liquid membrane, in a hunt for an efficient extraction method using a novel combination of carrier and strip solutions. A theoretical model was presented which explains the mass transfer in the form of metal-carrier complex. Maximum flux and permeability were observed by using TEDA as a carrier, HNO3 as feed and NaOH as a stripping agent. Selective complexation of the carrier and the desired metal ions is necessary for its removal, so preliminary extraction experiments were performed to sort out the credibility of the carrier. Finding an appropriate solvent for extraction is a challenge faced widely. The behavior of the carrier and its complex with the desired metal ion is greatly influenced by their interaction with the chosen solvent. The behaviors of different solvents were observed and CCl4 was defined to be appropriate for the present study. Concentration and other properties of the feed and stripping are essential in interface reactions involved in the transport mechanism, therefore, optimum conditions for strip and feed were sorted out experimentally. Parameters like flux and permeability were also studied. A literature survey showed no previous information on the extraction of Cd(II) using the aforementioned set of carrier, feed, and stripping.
Theory
The metal ions transport through the medium of supported liquid membrane involves the formation of metal-extractant complex, followed by its diffusion across the feed-membrane interface to the strip-membrane interface, as a function of a concentration gradient. Once it is transported across the membrane, metal-carrier complex degradation occurs. A theoretical model was proposed which governs the mass transfer of the Cd(ΙΙ) ions in the form of metal-carrier complex through diffusion.
Representing Triethylenediamine (TEDA) by symbol “A”, the following reactions have been proposed to occur.
A + H+ = AH+ (1)
According to our proposed mechanism, Cd(NO3)2 present in the feed solution undergoes a change due to excess of HNO3 present in the solution, as presented in the following equation.
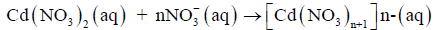 (2)
(2)
The interaction of these oppositely charged ions, i.e., (AH+) and [Cd(NO3)n+1]n-(aq) results in the formation of a complex at the feedmembrane interface:
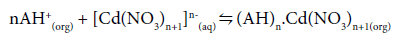 (3)
(3)
Eq. (3) may be represented in terms of the roles played by H+ and NO3-, as:
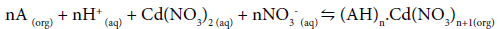 (4)
(4)
Reactions at strip membrane interface
As shown in eq. (3) and (4), the reaction of oppositely charged ions leads to the neutral complex formation which gets transported across the feed-membrane interface to strip-membrane interface. It is interesting to note that the neutral complex can easily be extracted in the organic phase. The dissociation of the complex at the strip-membrane interface is governed by NaOH present in the stripping phase as follows:
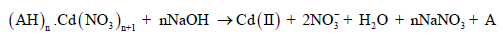 (5)
(5)
The carrier molecule (A) undergoes reverse diffusion across the liquid membrane, once it gets liberated after the decomposition of the complex, after which it re-plays the process of transport of a metal ion through complex formation. According to Wilke-Chang, the diffusion coefficient of complex (AH)n.Cd(NO3)n+1 in the forward direction is quiet lesser than that of the free carrier (A) in the backward direction, which justifies the proposed hypothesis of higher TEDA concentration at the feed-membrane interface when compared with the concentration of the complex.
The following equation shows the equilibrium constant KCd for Cd(II):
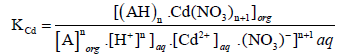 (6)
(6)
The distribution coefficient (λCd) which governs the distribution of Cd2+ between the membrane and aqueous phases have been shown as:
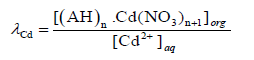 (7)
(7)
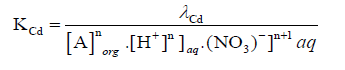 (8)
(8)
And on re-arranging Eq. (8)
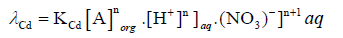 (9)
(9)
Considering the extraction constant, distribution coefficient and laws of diffusion, an equation can be derived for the flux (J) as shown:
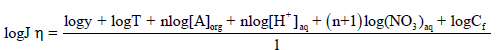 (10)
(10)
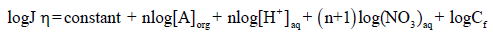 (11)
(11)
The Eq. (10) shows that the flux (J) of Cd2+ is directly proportional to the concentration of nitrate (NO3-), proton concentration (H+), carrier (A) and Cf, at a constant temperature. This equation is useful in calculating the number of protons present in AH+ form by retaining [NO3-], [A] and Cf constant in Eq. (11) and plotting log Jη vs. log [H+]. The resulting slope will be equal to the number of H+ ions present in the complex. Similarly, a slope of the plot of log Jη versus log [A] shows that 4.0 moles (n) of TEDA contributes in the formation of a complex with Cd(II), when [NO3-], [H+] and Cf are kept constant.
Materials and Methods
Chemicals and reagents
For investigating the transport of Cd(II), triethylamine (AR grade Applichem) dissolved in carbon tetrachloride (AR grade BDH) was employed as a carrier in the liquid membrane phase, HNO3 (64% Merck) as feed solution whereas NaOH (AR grade BDH) was used as a stripant for Cd(II) recovery.
Apparatus and instruments
Atomic absorption spectrometer, Perkin Elmer 400 with graphite furnace (resolution 0.001 ppm, accuracy ± 0.1 ppm), was used for measuring the Cd(II) concentration in aqueous phases. The wavelength and slit width were adjusted as follows: for Cd(II) 285.148 nm, 1.7/1.08 mm.
Neo met pH meter (resolution 0.001 pH, accuracy ± 0.003 pH) was used to find the pH of the feed and stripping solution.
Preparation of supported liquid membrane
For the present study, Celgard 2400 and 2500 hydrophobic, polypropylene films were chosen which acted as solid support for the SLM.
Membrane cell
The Cd(II) extraction study was performed in a permeation cell, which was constructed using Perspex material. The volume capacity of each of the cell was 0.25 dm3. Two cells or compartments which carried the membrane between them were joined and fixed with flanges. A rubber ring inert to the solution was placed on the membrane film in order to stop the leakage and mixing of the solution. The two different solutions present in both the compartments were constantly agitated at 1200 rpm at a temperature of 25 ± 1°C.
Permeation study
After mounting the compartment of the permeation cell and fixing the SLM between them, the feed and stripping solutions (0.25 dm3 each) were added simultaneously, covered and set on constant agitation. Uniform stirring was done to ensure a uniform concentration throughout the cell, and hence a concentration polarization was avoided at the membrane interfaces. A known quantity (1 cm3) was collected and analyzed for the metal ion concentration, from both the feed and the stripping phases after regular intervals.
The following equations were used to find out the flux (J) and permeability (p):
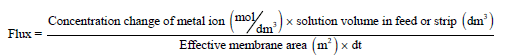 (12)
(12)
 (13)
(13)
Where Δt represents the total permeation time interval (in seconds).
Results and Discussion
Effect of HNO3 concentration in the feed phase
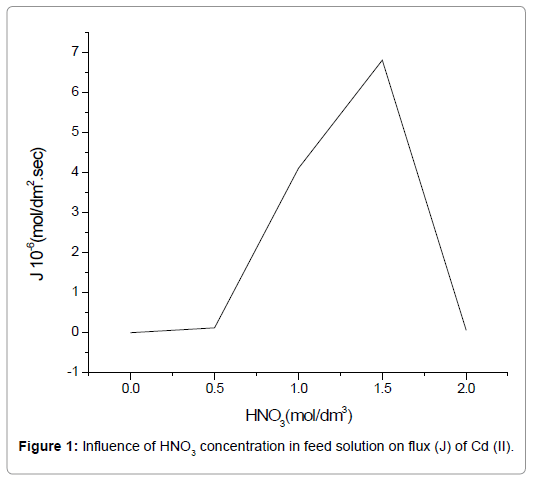
Nitric acid (HNO3) holds an important role in the extraction procedure because of both the proton and the nitrate ion, resulting from its dissociation. Both the H+ and NO3- are essential in the complex formation (LH)n.Cd(NO3)n+1) as per Eq.(4). The effect of its concentration on the extraction procedure was studied using a solution of varying molarity (0.5 mol/dm3 to 2.0 mol/dm3), keeping the TEDA concentration at a controlled value of 3.75 mol/dm3 in the liquid membrane phase. The NaOH concentration in the stripping phase was maintained at 1.5 mol/dm3. Figure 1 shows an increase in the flux of Cd(II) with an increase in the concentration of HNO3 from 0.5 mol/dm3 to 1.5 mol/dm3. An increased HNO3 concentration leads to increased availability of H+ and NO3-and hence increased complex formation as per Eq. (4), which ultimately increases the transport of Cd(II). An HNO3 concentration higher than 1.5 mol/dm3 results in a lower transport efficiency of Cd(II), which is due to the formation of Hn.Cd(NO3)n-type species. The aforementioned species are formed when H+ and NO3- are present in excess in the feed and therefore, reaction (3) is inhibited in the forward direction. Further studies for Cd(II) extraction were carried out using the optimum HNO3 concentration of 1.5 mol/dm3, and hence investigation of other essential parameters was done.
Figure 1. Influence of HNO3 concentration in feed solution on flux (J) of Cd (II).
Effect of stripping phase concentration on the permittivity of Cd(II)
The complex ((AH)nCd(NO3)n+1) is dissociated at the stripmembrane interface by the strippant. For studying the effect of varying NaOH concentration on the transport of Cd(II), various concentrations in the range of 0.5 mol/dm3 to 2.0 mol/dm3 were used, while maintaining the constant TEDA concentration at 3.75 M and HNO3 concentration in feed solution at 1.0 mol/dm3.
The Figure 2 shows that as the concentration of NaOH increases from 0.5 mol/dm3 to 1.5 mol/dm3, the permeability of Cd(II) increases, this is because of an increase in the OH- concentration in the strip phase which facilitates the complex break-down as per Eq. (v). Furthermore, when NaOH concentration is increased beyond 1.5 mol/dm3, the transport of Cd(II) decreases, this is because of the formation of an insoluble precipitate of Cd(OH)2 at a higher OH- concentration.
Sd2+ (aq) + 2OH- (aq) → Cd (OH)2(s) (14)
Figure 2. Effect of NaOH concentration in strip solution on permittivity (p) of Cd (II).
The precipitate blocks the pores of the polypropylene membrane and therefore Cd(II) extraction decreases.
Influence of stripping phase concentration on the flux of Cd(II)
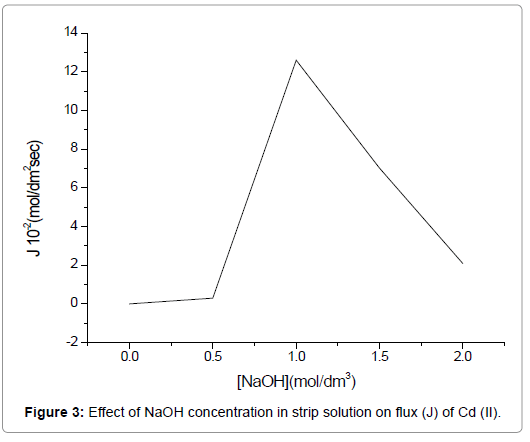
The strippant dissociates the complex ((AH)nCd(NO3)n+1) at the strip-membrane interface. To investigate the effect of varying concentrations of NaOH on Cd(II) extraction, a number of concentrations in the range of 0.5 mol/dm3 to 2.0 mol/dm3 were used, whereas the TEDA and HNO3 concentrations were kept constant at 3.75 M and 1.0 mol/dm3, respectively in the feed.
In Figure 3, an increase in the flux (J) of Cd(II) could be observed with the increase in the concentration of NaOH from 0.5 mol/dm3 to 1.0 mol/dm3, which is justified on the basis of increased availability of OH- and hence enhanced complex breakdown at stripping membrane interface occurs as per Eq. (5). However, a further increase in NaOH concentration beyond 1.0 mol/dm3 results in a lower Cd(II) transport. Hence it may be ascribed to the production of large quantity of OHions, which get H+ ions from carrier to restrict further transport.
Figure 3. Effect of NaOH concentration in strip solution on flux (J) of Cd (II).
Effect of feed concentration on the flux of Cd(II)
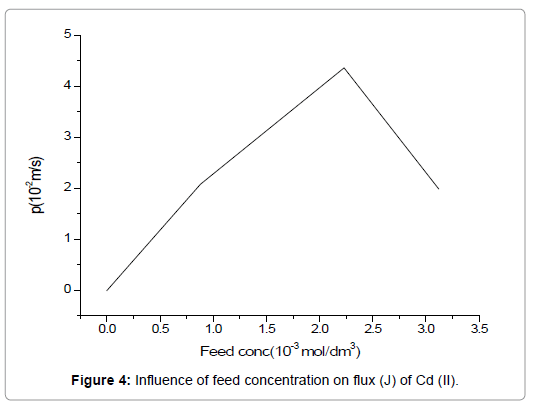
Different concentrations of Cd(II) ranging from 1.99 × 10-2 mol/dm3 to 4.36 × 10-2 mol/dm3 were used as feed solutions, in investigating the efficiency of the supported liquid membrane for metal ions extraction. For the present work, the concentration of carrier was kept constant (3.75 M) whereas, stripping phase concentration and HNO3 concentration in the feed solution was maintained at 1.5 mol/dm3 and 1.0 mol/dm3 respectively.
Figure 4 indicates an increase in the permeability of Cd(II) with an increase in its concentration in the feed as per Eq. (10), where flux (J) is directly proportional to feed concentration (Cf). This study shows that at a metal ion concentration of 2.2 × 10-3 mol/dm3, the permeability is maximum i.e., 4.3 × 10-3 and gradually decreases to 2.1 × 10-3 at 3.2 × 10-3 mol/dm3. This decrease in the permeability, in a relationship with a higher metal ion concentration, may be explained on the basis of saturation of carrier and a decrease in the effective membrane surface. Additionally, the formation of the complex cause super-saturation of the membrane by forming a layer on the membrane interface and hence Cd(II) extraction is minimized.
Figure 4. Influence of feed concentration on flux (J) of Cd (II).
The flux and permeability values obtained were reproducible with an average error of ±12%.
Effect of membrane thickness on the extraction of Cd(II)
The influence of membrane thickness on the extraction of Cd(II) was studied, using microporous hydrophobic membranes of varying Celgard i.e., 2400, 2500 and 4400. Figure 5 shows an inverse relationship between the flux of Cd(II) and the thickness of the membranes. The thickest membrane of Celgard 4400 allows the lowest flux of 1.01 × 10-4 mol/m2s. The obtained results condone the proposed theoretical model where the flux and thickness of membrane have inverse relation as shown in Eq. (10).
Figure 5. Effect of the membrane thickness on flux (J) of Cd (II).
Conclusion
Cd(II) ions were extracted across TEDA-carbon tetrachloride Supported Liquid Membrane. A theoretical model was proposed, suggesting the formation of metal-extractant complex, diffusion through the interface, followed by the degradation of the complex in the strip solution. The optimum parameters based on the theoretical model matched well with the experimental values. It was concluded that one mole of each TEDA and protons interact with Cd(II) and result in the formation of [(AH)n.Cd(NO3)n+1 (org) type of complex which is responsible for the transport of Cd(II) ions. Therefore 1.0 M of HNO3 in the feed solution, 1.5 M in stripping phase, 3.75 M of TEDA in membrane phase and 2.36 × 10-3 mol/dm3 of Cd(II) metals ions concentrations were found to be the optimum conditions for the transport of Cd(II). The membrane thickness was found to have an inverse relation with the extraction process. The flux improved with increasing concentrations of NaOH from 0.5 to 1.0 mol/dm3 and declined back on the further increase, whereas permeability was observed to increase with an increase of the feed concentration till 2.23 × 10-3 mol/dm3, followed by a decline with the further increase due to maximum saturation of carrier.
REFERENCES
- Smuleac V, Butterfield D, Sikdar S, Varma R, Bhattacharyya D (2005) Polythiol-functionalized alumina membranes for mercury capture. J Membr Sci 251: 169-178.
- Dozol JF, Dozol M, Macias RM (2000) Extraction of strontium and cesium by dicarbollides, crown ethers and functionalized calixarenes. J Incl Phenomena Macrocycl Chem 38: 1-22.
- Matsunga T, Takeyama H, Nakao T, Yamazawa A (1999) Screening of marine microalgae for bioremediation of cadmium-polluted seawater. J Biotechnol 70: 33-38.
- Ulewicz M, Lesinska U, Bochenska M, Walkowiak W (2007) Facilitated transport of Zn(II), Cd(II) and Pb(II) ions through polymer inclusion membranes with calix [4]-crown-6 derivatives. Sep Purif Technol 54: 299-305.
- Jyotsna G, Kadirvelu K, Rajagopal C (2004) Competitive sorption of Cu(II), Pb(II) and Hg(II) ions from aqueous solution using coconut shell-based activated carbon. Ad Sci Technol 22: 257-273.
- Marchioretto MM, Bruning H, Rulkens W (2005) Heavy metals precipitation in sewage sludge. Sep Sci Technol 40: 3393-3405.
- Stamatialis DF, Paprnburg BJ, Girones M, Saiful S, Bettahalli SNM, et al. (2007) Medical applications of membranes: Drug delivery, artificial organs and tissue engineering. J Membr Sci 308: 1-34.
- He D, Gu S, Ma M (2007) Simultaneous removal and recovery of cadmium(II) and CN-from simulated electroplating rinse wastewater by a Strip Dispersion Hybrid Liquid Membrane (SDHLM) containing double carrier. J Membr Sci 305: 36-47.
- Zaghbani A, Tayeb R, Bonnamour I, Felix C, Vocanson F, et al. (2005) Affinity membranes for the extraction of Cd2+ metal ions by facilitated transport ensured by a new thiacalix [4] arene complexing agent incorporated in Supported Liquid Membranes (SLM). J Membr Sci 258: 5-7.
- Danesi P, Chiarizia R, Castagnola A (1983) Transfer rate and separation of Cd(II) and Zn(II) chloride species by a trilaurylammonium chloride-triethylbenzene supported liquid membrane. J membr Sci 14: 161-174.
- Breembroek GRM, Van Straalen, A, Witkamp GJ, Van Rosmalen GM (1998) Extraction of cadmium and copper using hollow fiber supported liquid membranes. J Membr Sci 146: 185-195.
- Claudia F, Manuela H, Victoria S, Enriqueta A (2005) Selective recovery and preconcentration of mercury with benzoylthiourea-solid supported liquid membrane system. Anal Chim Acta 547: 255-261.
- Junwen Lv, Yang Q, Jiang J, Chung TS (2007) Exploration of heavy metal ions transmembrane flux enhancement across a supported liquid membrane by appropriate carrier selection. Chem Eng Sci 62: 6032-6039.
- Daoud JA, El-Reefy A, Aly HF (1998) Permeation of Cd(ll) Ions through a Supported Liquid Membrane Containing Cyanex-302 in Kerosene. Sep Sci and Technol 33: 537-549.
- Rathore NS, Leopold A, Pabby AK, Fortuny A, Coll MT, et al. (2009) Extraction and permeation studies of Cd(II) in acidic and neutral chloride media using Cyanex 923 on supported liquid membrane. Hydrometallurgy 96: 81-87.
- Nowier HG, El-Said N, Aly HF (2000) Carrier-mediated transport of toxic elements through liquid membranes Transport of Cd(II) from high salinity chloride medium through supported liquid membrane containing TBP/cyclohexane.J Membr Sci 177: 41-47.
- Altin S, Alemdar S, Altin A, Yildirim Y (2011) Facilitated transport of Cd(II) through a supported liquid Membrane with Aliquat 336 as a carrier.Sep Sci Technol 46: 754-764.
- He D, Ma M, Zhao Z (2000) Transport of cadmium ions through a liquid membrane containing amine extractants as carrier.J Membr Sci 169: 53-59.
Citation:
Bibi N, Rehman HU, Khan K, Kanwal A, Gul R, Ali K, et al. (2019) Extraction and Facilitated Transport of Cd(II) Ions across Triethylenediamine-Carbon Tetrachloride Supported Liquid Membranes. J Membr Sci Technol 9:194.
Copyright:
© 2019 Bibi N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.