Indexed In
- Open J Gate
- JournalTOCs
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2019) Volume 7, Issue 1
Evaluation of the Acute and Sub-chronic Toxicity of the Ethanolic Extract of Curcuma longa (Zingiberaceae) in Wistar Albino Rats
Donatien Gatsing1*, Gabriel Tchuente Kamsu1, Simeon Pierre Chegaing Fodouop2, Richard Simo Tagne2, Norbert Kodjio1 and Adolette Lesly Nguelebek Fakam12Department of Biomedical Sciences, University of Ngaoundéré, PO. Box 454, Ngaoundéré, Cameroon
Received: 06-Nov-2019 Published: 04-Dec-2019
Abstract
Background: Commonly called "two hundred diseases" in Western Cameroon, Curcuma longa is a plant widely used in traditional medicine for many years. In order to evaluate the safety of this plant commonly use in the treatment of typhoid fever in Cameroon, the acute and Sub-chronic toxicity of the ethanolic extract of Curcuma rhizomes has been studied in albino rats of Wistar strain.
Materials and methods: Acute and sub-acute toxicity of this extract were study using OCDE 2008 guidelines.
Results: The physical parameters were recorded during the experiment and the hematological and biochemical parameters, as well as the histology of the liver and reproductive organs were carried out at the end of the experiment. The acute toxicity study revealed no toxic effects, no behavioural disturbances and no death in rats. The Sub-chronic toxicity study revealed an increase in feed intake and weight gain in male and female rats at test doses throughout the treatment period. A significant decrease of transaminase activity, serum creatinine level, LDL cholesterol level and the artherogenicity index in the test animals compared with the control were noted. Repeated administration of the extract also increased urinary creatinine levels, white blood cell counts, and HDL cholesterol levels. The extract kept a constant level of red blood cells in animals and had no visible effect on the reproductive organs of male and female rats.
Conclusion: This extract can therefore be used for the formulation of phytomedicine against typhoid fever at the therapeutic dose of 30 mg/kg used by traditional practitioners.
Keywords
Curcuma longa; Ethanolic extract; Toxicological evaluation; Histology
Introduction
The use of plants for their medicinal benefits is constantly increasing because it plays a very important role in the treatment of many human diseases [1]. However, studies done over the last 20 years have shown that not all-natural substances are absolutely good for health. Although the frequency of poisoning by plants is poorly known in tropical and intertropical zones, because of the absence of anti-poison centres, the incidence of mortality linked to poisoning by plant consumption is still known in some European countries [2]. These poisonings are generally due to the presence in these plants of toxic compounds whose main target organs are the liver, kidneys, hematopoietic cells, reproductive organs and many others. The urgency is therefore in the preliminary study of the possible risks that the extracts of medicinal plants would bring to the host who consumes them, in order to increase the safety of their use.
Curcuma longa is a perennial herb, a member of the Zingiberaceae family and known by several names such as, turmeric (English name), yellow Djindja (Cameroon). It is very rich in phenolic compounds (Curcumin). It is cultivated in India, China and other tropical regions [3] and the part commonly used is its rhizome. The rhizomes of C. longa have been used in traditional medicine for many years and in many countries to cure human diseases. The effectiveness and frequency of its use is at the origin of the name 200 diseases that has been attributed to this plant in Cameroon.
It is traditionally used in the West region of Cameroon for the treatment of several diseases such as typhoid fever [4]. Ethanolic extract and many other organic solvent extracts have shown significant results in vitro and in vivo against salmonellosis [5-7]. According to Kodjio et al. 30 mg/kg and 60 mg/kg were the recommended doses for the effective treatment of typhoid fever infected rats. In order to contribute to the management of patients suffering from typhoid fever, toxicological studies concerning this ethanolic extract of C. longa in repeated administration are essential. This study therefore conditions the production of improved traditional medicines against typhoid fever, which continues to be a public health problem.
Material and Methods
Plant materials
The medicinal plant used in this study is Curcuma longa rhizomes. These rhizomes were collected in Santchou, Menoua Division, West Region of Cameroon in March 2017. These plants were authenticated at the Cameroon National Herbarium, under the number 42173/HNC by presentation of a specimen.
Preparation of the extract
After harvest, the rhizomes of C. longa were washed, cut, dried at room temperature away from the sun and ground to obtain à powder using a Moulinex brand Zaiba (Super Blender, China). Nine hundred grams (900 gms) of powder were macerated in 4 L of ethanol 95% for 48 hours at room temperature with occasional shaking. The extract was filtered through a Whatman No: 1 filter paper. The filtrate was dried in a ventilated oven (Memmert) set at 40°C until complete evaporation of the solvent. The crude extract was kept at 4°C in a sterile and impervious bottle until further use.
Experimental animals
Forty-six Wistar albino rats were used, 6 females for acute toxicity and 20 males and 20 females for Sub-chronic toxicity. These rats were aged between 08 and 09 weeks and weighing between 140 and 200 g. The animals were raised at the animal house of University of Dschang and housed individually in polypropylene cages at 23 ± 1ºC in 12:12 h dark: light cycle. Food and tap water were giving ad libitum. The animals were treated according to the protocol for the use of laboratory animals, based on the ethical considerations of the Government of India Animal Monitoring and Supervision Committee (Registration No. 173/CPCSEA, dated of 28 January 2000, of the Government of India, on the use of animals for scientific research).
Acute toxicity
The OECD guideline 425 on the dose adjustment method [8] was used. In this test, six (6) nulliparous and non-pregnant rats aged 8 weeks and weighing between 140 and 150 g were used. The three (3) control animals received the vehicule (distilled water) and the other three received the extract at a dose of 5000 mg/kg by gavage using an endogastric tube, one animal after the other, and in the time intervals of 48 hours. The trial ended when three serial rats survived the 5000 mg/kg dose. In order to detect certain toxic effects, each rat was observed with particular attention during the first 3 hours following the administration of the extract and regularly during the first 24 hours; then daily for 14 days. After three hours of observation, the rats had free access to food and water. Different physical symptoms of toxicity (changes in autonomic or behavioral responses (locomotion, aggressiveness), spontaneous activity (reaction to tail pinch and to noise), social interactions, corneal reflex, aspect of faces and mortality [8,9] have been evaluated. After 14 days of observation, each animal was subjected to a macroscopic autopsy to identify any macroscopic pathological changes in organs such as the liver, kidneys, lungs and heart. The organs of animals treated with the extract were compared with those of control animals [8].
Sub-chronic toxicity
Repeat-dose oral toxicity study was carried out according to OECD guideline 407 [10]. Forty (40) rats including 20 males and 20 females 9 weeks old (weighing between 140 and 200 g) were randomly assigned to 5 groups of 4 rats per group and sex. Groups 1 to 4 received 1.5 ml of extract at doses of 30 mg/kg, 60 mg/ kg, 120 mg/kg and 240 mg/kg body weight respectively during 28 day. The control group (batch 5) received only 1.5ml 5% DMSO. During these 28 days extract was administered daily at the same time. Food and water intake as well as body weights of the animals were recorded daily.
Determination of food intake: Food consumption was determined daily by differentiating between the amounts of food (in gm) deposited the day before and the quantities remaining the next day.
Determination of weight gain or loss: The increase or loss of weight was evaluated daily and the gain or loss of weight was determined by the following formula:
W=Wf - W0
Where: W=Gain or loss of growth at day x; Wf=Body weight of the animal at day x; W0=Initial body weight of the animal (before the start of the test).
Sacrifice of animals and collection of blood and organs: On the 28th day of the test, after gavages, the animals were subjected to a 12-hours food fast at the end of which the urine was collected. Subsequently, the animals were anesthetized with chloroform vapour, then placed in a supine position and the four limbs were immobilized. A dissection was performed on the abdomen and the blood was collected by cardiac puncture and then first introduced into a tube containing an anticoagulant (EDTA) for hematological analysis and secondly into a tube without anticoagulant for the preparation of the serum. Then, certain organs such as the heart, kidneys, liver, lung, spleen and testis/ovaries were immediately removed. These organs were used to evaluate the relative weight of organs. One part of each organ for histopathological analyzes was stored in 10% buffered formalin and the other part was milled to obtain the homogenate for total tissue protein assay.
Hematological analysis: Hematological analyses were performed by a blood count using an impedance hematology automaton (QBC Autoread Plus, UK).
Biochemical analysis: On the 28th day of experiment, animals were subjected to overnight fasting during which their urine was collected used for the determination of creatinine and total urinary protein (Biuret method) using commercial SG Mitalia brand kits (Italy). Then after, the blood collected by cardiac puncture into heparinized and non-heparinized tubes. Tubes without anticoagulant was used to prepare the serum by centrifuging them at 3000 rpm for 15 min. serum obtained was used to determine biochemical parameters such as creatinine, Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), Total Cholesterol, High Density Lipoprotein (HDL), Triglycerides and Total Proteins were using commercial SG Mitalia brand kits (Italy).
Animals were further dissected and gross pathological examinations were done including weighing of different organs (liver, kidney, testis and ovary). Fifteen percent homogenate of these organs were prepared in a phosphate buffer solution (pH 7.4) at the rate of 15 g of tissue per 100 ml of buffer and centrifuged at 3000 rpm for 15 minutes. The supernatant was recovered for determination of the total tissue proteins (Biuret method) with the kits of the same brands.
Histopathological analysis: The liver, kidney, testis and ovary of the test rats, previously preserved in 10% formalin, were subjected to histological techniques by cutting a thickness of 5 μm using a microtome, followed by staining with hematoxylin-eosin and the slides were observed under a light microscope equipped with camera for the realization of photographs [11].
Statistical analysis
Statistical analysis was performed using SPSS versions 20.0 for Windows. The results were expressed as mean value ± standard error of mean (S.E.M.) and the comparisons were performed by the analysis of variance using ANOVA test. Differences between averages of control and drug treated groups where they existed were separated using the Waller-Duncan test. A probability value of less than 0.05 was fixed as the statistical significance criterion.
Results
Acute toxicity
No mortality or abnormality was recorded in female rats at the single dose of 5000 mg/kg body weight. The general behaviour of the extract-treated animals and the control showed that no changes related to the extract were observed in the behaviour of the test rats, apart from the slight drowsiness during the 30 minutes following administration of the extract. Macroscopic examination of the liver, heart, kidneys, spleen and lungs revealed no abnormalities in treated rats compared to the control rats after the observation period.
Sub-chronic toxicity
Food consumption and weight gain: Administration of the ethanol extract of C. longa caused an increase in food intake of both sexes compared to control animals throughout the test period. This increase of the weight gain has resulted to an increase of food intake of rats of both sexes compared to controls during the 28 days of testing (Figure 1).
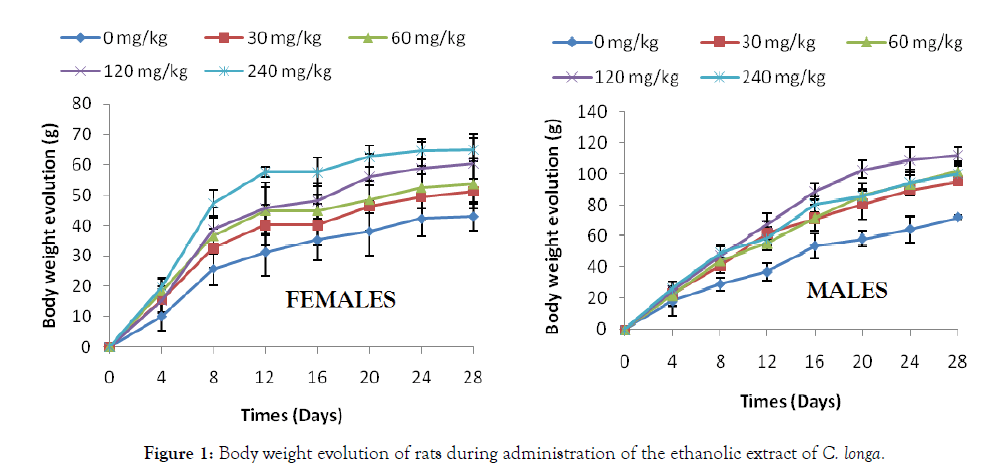
Figure 1: Body weight evolution of rats during administration of the ethanolic extract of C. longa.
Relative weight of organs: Table 1 shows the results of the relative weight of organs after repeated administration of ethanolic extract of C. longa to female and male rats. It shows that no significant variation (p ≥ 0.05) in organ weights was observed.
| Sex | Doses (mg/kg) | Liver (g) | Heart (g) | Kidneys (g) | Spleen (g) | Lungs (g) | Ovaries /Testis (g) |
|---|---|---|---|---|---|---|---|
| Females | 0 | 2.97 ± 0.05a | 0.31 ± 0.02a | 0.59 ± 0.11a | 0.30 ± 0.02a | 0.57 ± 0.08a | 0.03 ± 0.02a |
| 30 | 3.11 ± 0.22a | 0.30 ± 0.01a | 0.64 ± 0.03a | 0.28 ± 0.06a | 0.58 ± 0.08a | 0.03 ± 0.00a | |
| 60 | 3.10 ± 0.15a | 0.30 ± 0.03a | 0.64 ± 0.02a | 0.25 ± 0.03a | 0.54 ± 0.02a | 0.03 ± 0.00a | |
| 120 | 3.10 ± 0.28a | 0.30 ± 0.04a | 0.65 ± 0.04a | 0.23 ± 0.04a | 0.46 ± 0.07a | 0.03 ± 0.00a | |
| 240 | 3.10 ± 0.28a | 0.29 ± 0.02a | 0.71 ± 0.12a | 0.28 ± 0.11a | 0.54 ± 0.07a | 0.02 ± 0.00a | |
| Males | 0 | 3.09 ± 0.04a | 0.33 ± 0.03a | 0.61 ± 0.10a | 0.31 ± 0.03a | 0.59 ± 0.08a | 0.93 ± 0.10a |
| 30 | 3.18 ± 0.32a | 0.32 ± 0.01a | 0.65 ± 0.03a | 0.29 ± 0.06a | 0.58 ± 0.08a | 0.93 ± 0.45a | |
| 60 | 3.10 ± 0.25a | 0.32 ± 0.04a | 0.65 ± 0.01a | 0.26 ± 0.04a | 0.58 ± 0.02a | 0.90 ± 0.03a | |
| 120 | 3.28 ± 0.22a | 0.32 ± 0.04a | 0.65 ± 0.02a | 0.28 ± 0.04a | 0.58 ± 0.07a | 0.88 ± 0.08a | |
| 240 | 3.19 ± 0.26a | 0.31 ± 0.03a | 0.70 ± 0.12a | 0.29 ± 0.10a | 0.60 ± 0.07a | 0.87 ± 0.10a |
Table 1: Effect of different doses of C. longa extract on the relative weight of organs.
Effect of extracts on hematological parameters: Table 2 shows the change in total white and red blood cell count and their respective figurative elements in animals treated with different doses of C. longa ethanolic extract. From the analysis of this table, it appears that the extract generally resulted in a significant (p<0.05) increase in the level of white blood cells, lymphocytes, monocytes, granulocytes in the test animals compared to the controls. The analysis of this table also reveals that repeated administration to different doses of the extract of this plant did not result in any significant changes in red blood cell count in test animals of both sexes when compared to controls.
| Sex | Parameters Studied | 0 mg/kg | 30 mg/kg | 60 mg/kg | 120 mg/kg | 240 mg/kg |
|---|---|---|---|---|---|---|
| Females | WBC (× 103/μl) | 4.36 ± 0.77a | 4.33 ± 0.15a | 4.13 ± 0.32a | 5.13 ± 0.73ab | 6.26 ± 0.47b |
| RBCs (× 106/μl) | 7.57 ± 0.24a | 7.71 ± 0.11a | 7.84 ± 0.31a | 7.91 ± 0.14a | 7.67 ± 0.27a | |
| Lymphocytes (%) | 81.00 ± 1.82a | 86.50 ± 1.21ab | 83.26 ± 3.54a | 86.33 ± 2.43ab | 81.76 ± 5.85a | |
| Monocytes (%) | 3.06 ± 0.65a | 2.53 ± 0.61a | 3.23 ± 0.70a | 2.90 ± 0.55a | 3,36 ± 0,37a | |
| Granulocytes (%) | 5.60 ± 0.85a | 7.30 ± 0.52b | 9.83 ± 1,02b | 9.43 ± 0.81b | 9.86 ± 0.68b | |
| Platelets (× 103/μl) | 282.33 ± 3.15a | 272.00 ± 7.69a | 305.66 ± 6.10a | 295.00 ± 0.22a | 303.66 ± 1.50a | |
| Haemoglobin (g/dl) | 15.36 ± 0.51a | 15.90 ± 0.87a | 15.73 ± 0.55a | 15.90 ± 0.55a | 15.33 ± 0.40a | |
| Haematocrit (%) | 45.96 ± 3.40a | 46.90 ± 2.79a | 48.40 ± 0.60a | 44.90 ± 4.07a | 48.80 ± 1.03a | |
| Males | WBC (× 103/μl) | 5.56 ± 0.66a | 6.76 ± 0.64ab | 5.60 ± 0.79a | 7.53 ± 0.68b | 7.96 ± 0.30b |
| RBCs (× 106/μl) | 7.95 ± 0.63a | 8.08 ± 0.49a | 8.04 ± 0.26a | 8.16 ± 0.12a | 7.99 ± 0.74a | |
| Lymphocytes (%) | 81.20 ± 0.69a | 84.13 ± 1.84a | 91.53 ± 1.76b | 93.20 ± 1.58b | 92.26 ± 3.95b | |
| Monocytes (%) | 2.66 ± 0.30a | 3.36 ± 0.47ab | 3.60 ± 0.20b | 3.10 ± 0.60ab | 3.60 ± 0.34b | |
| Granulocytes (%) | 5.80 ± 0.40a | 6.16 ± 1.05a | 11.53 ± 0.92b | 10.70 ± 0.50b | 11.80 ± 0.36b | |
| Platelets (× 103/μl) | 283.00 ± 18.35a | 290.66 ± 11.01a | 318.33 ± 3.62a | 307.00 ± 10.14a | 302.00 ± 15.24a | |
| Haemoglobin (g/dl) | 16.56 ± 0.50a | 16.93 ± 0.56a | 16.66 ± 0.50a | 16.36 ± 0.47a | 17.50 ± 0.08a | |
| Haematocrit (%) | 49.03 ± 3.91a | 40.60 ± 10.74a | 52.53 ± 0.10a | 49.60 ± 1.92a | 45.00 ± 5.65a |
Table 2: Effect of C. longa extract on hematological parameters.
Effect of extracts on biochemical parameters: The effect of different doses of extract on biochemical parameters is shown in Table 3. The analysis in this table shows that repeated administration of different doses of C. longa extracts leads to a significant decrease (p<0.05) of serum transaminase (ALT and AST) activity in animals of both sexes relatively to the controls. Repeated administration of different doses of C. longa involved to a significant decrease (p<0.05) of serum creatinine compared to controls, whereas urinary creatinine increased significantly from the dose 60 mg/ kg. Repeated administration of the animals to the extract generally induced a significant decrease (p<0.05) of triglyceride, total cholesterol, LDL cholesterol and the atherogenic index compared to controls. Nonetheless, a significant increase in HDL cholesterol compared with controls was observed in both sexes compared to controls. The effect of prolonged administration on tissue protein levels, serum protein levels, and urine protein levels is shown in Table 4. Analysis of this table shows an increase in splenic and hepatic protein levels in treated animals of both sexes compared to controls at all doses. However, a significant decrease (p<0.05) in serum and urinary proteins was also observed at all doses compared to controls.
| Sex | Parameters studied | 0 mg/kg | 30 mg/kg | 60 mg/kg | 120 mg/kg | 240 mg/kg |
|---|---|---|---|---|---|---|
| Females | ALT (UI/L) | 23.57 ± 2.16c | 20.62 ± 1.86b | 21.82 ± 1.28bc | 19.86 ± 1.30b | 16.15 ± 0.94a |
| AST (UI/L) | 73.55 ± 6.74c | 41.24 ± 4.35a | 38.30 ± 2.05a | 32.84 ± 5.36a | 42.92 ± 4.94ab | |
| S-Crea (mg/dl) | 0.90 ± 0.75b | 0.90 ± 0.62b | 0.52 ± 0.56a | 0.37 ± 0.35a | 0.40 ± 0.14a | |
| UCrea (mg/dl) | 15.66 ± 4.19a | 21.75 ± 5.50a | 29.16 ± 9.07b | 28.00 ± 7.32b | 50.66 ± 4.30c | |
| Trg (mg/dl) | 110.08 ± 4.83b | 99.12 ± 1.1ab | 84.71 ± 0.79a | 82.51 ± 9.94a | 84.14 ± 0.45a | |
| T-Chol (mg/dl) | 142.87 ± 2.51d | 130.04 ± 3.19cd | 116.50 ± 5.40bc | 79.17 ± 7.40a | 96.12 ± 1.77ab | |
| HDL mg/dl) | 35.21 ± 2.85a | 51.82 ± 3.87b | 54.14 ± 2.86b | 49.85 ± 3.09b | 48.13 ± 5.37b | |
| LDL (mg/dl) | 85.64 ± 1.37d | 58.39 ± 4.66c | 45.42 ± 5.04bc | 10.41 ± 8.90a | 21.16 ± 2.72ab | |
| AI | 1.63 ± 0.12b | 1.38 ± 0.02a | 1.31 ± 0.05a | 1.38 ± 0.05a | 1.35 ± 0.07a | |
| Males | ALT (UI/L) | 34.26 ± 3.23c | 26.19 ± 0.94b | 23.78 ± 1.15b | 15.93 ± 2.37a | 14.95 ± 1.93a |
| AST (UI/L) | 56.63 ± 7.73b | 58.60 ± 11.06b | 46.70 ± 3.58ab | 40.48 ± 7.45a | 43.21 ± 2.85a | |
| S-Crea (mg/dl) | 1.52 ± 1.26b | 0.97 ± 0.45a | 0.90 ± 0.53a | 0.80 ± 0.39a | 0.87 ± 0.48a | |
| U-Crea (mg/dl) | 16.00 ± 9.12a | 23.75 ± 10.29b | 21.25 ± 7.50ab | 25.00 ± 7.14b | 51.75 ± 4.78c | |
| Trg (mg/dl) | 144.93 ± 6.09d | 123.12 ± 4.59c | 72.24 ± 8.11a | 97.13 ± 7.88b | 98.12 ± 5.35b | |
| T-Chol (mg/dl) | 190.64 ± 1.06c | 158.41 ± 7.57ab | 145.39 ± 3.78a | 178.01 ± 5.17bc | 173.57 ± 4.00bc | |
| HDL mg/dl) | 44.81 ± 4.01a | 55.97 ± 8.31ab | 55.55 ± 5.76ab | 65.14 ± 6.44b | 63.35 ± 7.44b | |
| LDL (mg/dl) | 116.84 ± 0.24b | 77.81 ± 1.24a | 75.38 ± 7.27a | 93.43 ± 0.49ab | 86,58 ± 5.23a | |
| AI | 1.65 ± 0.07c | 1.44 ± 0.08b | 1.26 ± 0.05a | 1.30 ± 0.04a | 1.37 ± 0.05ab |
Table 3: Effect of C. longa extract on biochemical parameters.
| Sex | Doses (mg/kg) | Spleens (mg/g) | Lungs (mg/g) | Kidneys (mg/g) | Hearts (mg/g) | Liver (mg/g) | Serum (g/dl) | Urine (g/dl) |
|---|---|---|---|---|---|---|---|---|
| Females | 0 | 0.99 ± 0.06a | 0.51 ± 0.07a | 0.97 ± 0.25a | 0.38 ± 0.03a | 1.10 ± 0.13a | 6.77 ± 0.33b | 0.72 ± 0.03c |
| 30 | 1.20 ± 0.20a | 0.53 ± 0.04a | 0.86 ± 0.01a | 0.39 ± 0.01a | 1.34 ± 0.07b | 6.82 ± 0.29b | 0.62 ± 0.07c | |
| 60 | 1.36 ± 0.05b | 0.54 ± 0.05a | 0.87 ± 0.05a | 0.44 ± 0.09a | 1.23 ± 0.03ab | 5.87 ± 0.43a | 0.56 ± 0.11bc | |
| 120 | 1.28 ± 0.27ab | 0.48 ± 0.11a | 0.85 ± 0.06a | 0.40 ± 0.03a | 1.41 ± 0.05bc | 5.71 ± 0.26a | 0.47 ± 0.10b | |
| 240 | 1.25 ± 0.15ab | 0.49 ± 0.10a | 0.93 ± 0.06a | 0.46 ± 0.03a | 1.62 ± 0.23c | 6.00 ± 0.23a | 0.30 ± 0.07a | |
| Males | 0 | 1.71 ± 0.07a | 0.85 ± 0.17a | 1.39 ± 0.06a | 0.64 ± 0.05a | 1.57 ± 0.16a | 6.22 ± 0.54c | 0.87 ± 0.03c |
| 30 | 1.68 ± 0.08a | 0.75 ± 0.10a | 1.86 ± 0.18a | 0.69 ± 0.06a | 1.84 ± 0.10b | 5.97 ± 0.49bc | 0.70 ± 0.04b | |
| 60 | 1.70 ± 0.27a | 0.72 ± 0.07a | 1.69 ± 0.17a | 0.70 ± 0.03a | 1.69 ± 0.05ab | 5.58 ± 0.50b | 0.32 ± 0.06a | |
| 120 | 2.85 ± 0.21b | 0.74 ± 0.15a | 1.82 ± 0.08a | 0.71 ± 0.04a | 1.84 ± 0.19b | 5.62 ± 0.39b | 0.35 ± 0.05a | |
| 240 | 2.59 ± 0.07b | 0.77 ± 0.11a | 1.52 ± 0.08a | 0.66 ± 0.04a | 1.81 ± 0.15b | 5.09 ± 0.44ab | 0.44 ± 0.07a |
Table 4: Effect of C. longa extract on the tissue, serum and urine protein levels of animals.
Histological sections of the liver, kidneys and testes or ovaries: A repeated 28-day administration of the ethanolic extract of C. longa rhizomes did not cause any adverse effects on the liver, kidney, testicular and ovarian tissues of the test rats (Figures 2-7).
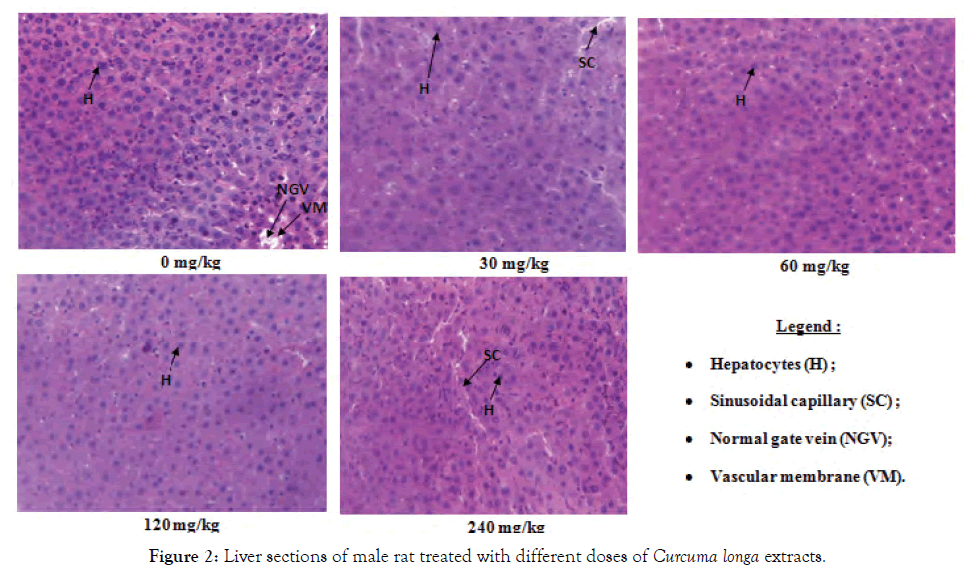
Figure 2: Liver sections of male rat treated with different doses of Curcuma longa extracts.
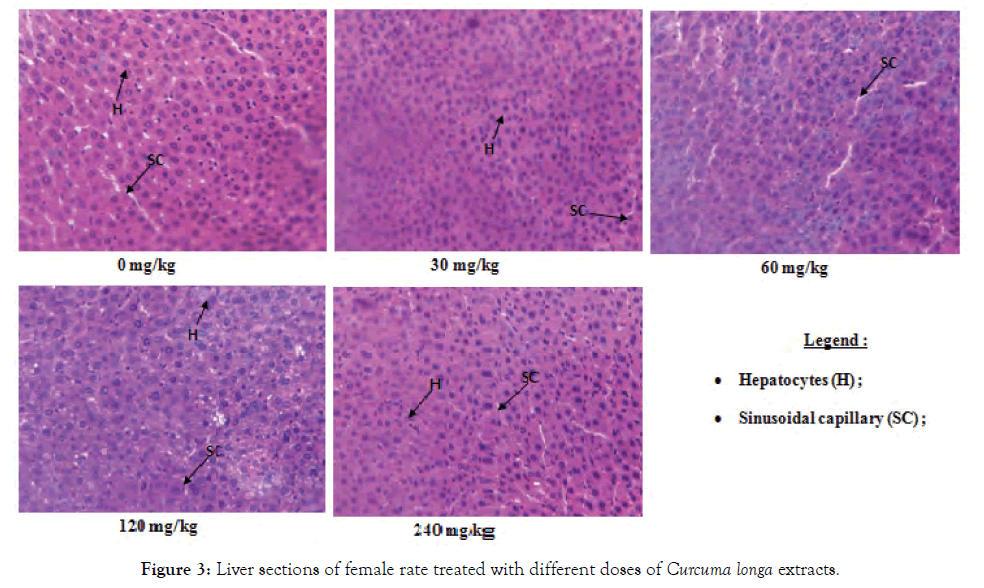
Figure 3: Liver sections of female rate treated with different doses of Curcuma longa extracts.
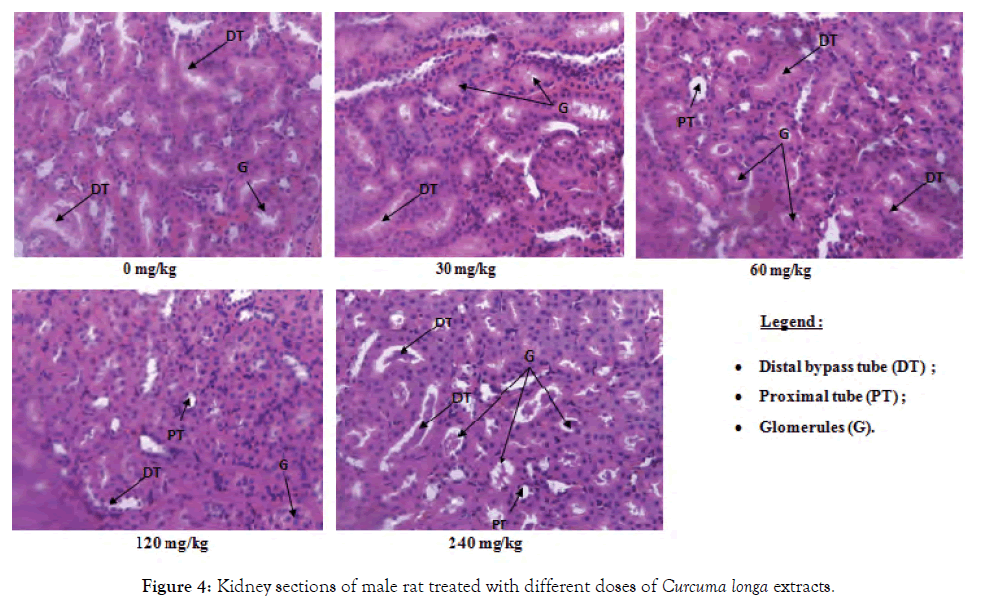
Figure 4: Kidney sections of male rat treated with different doses of Curcuma longa extracts.
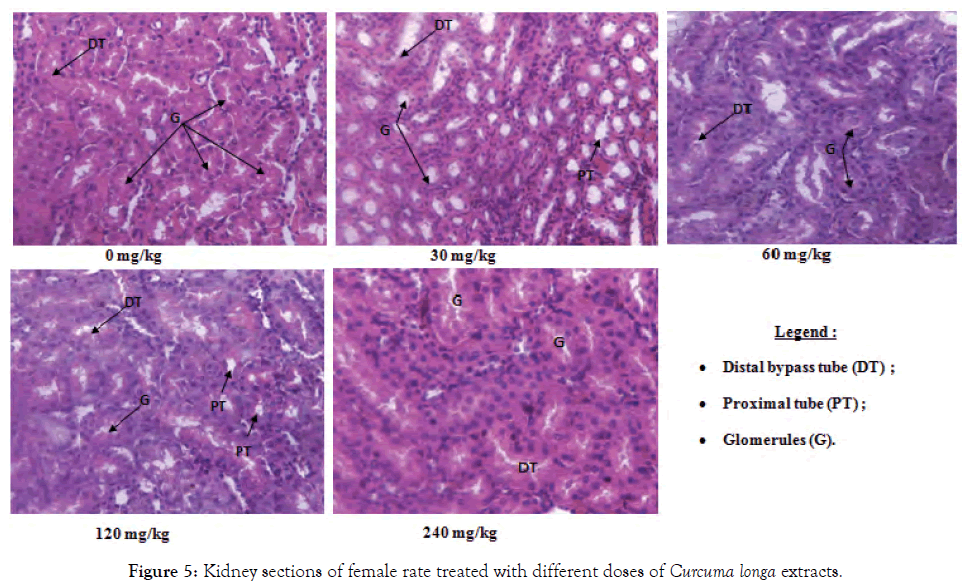
Figure 5: Kidney sections of female rate treated with different doses of Curcuma longa extracts.
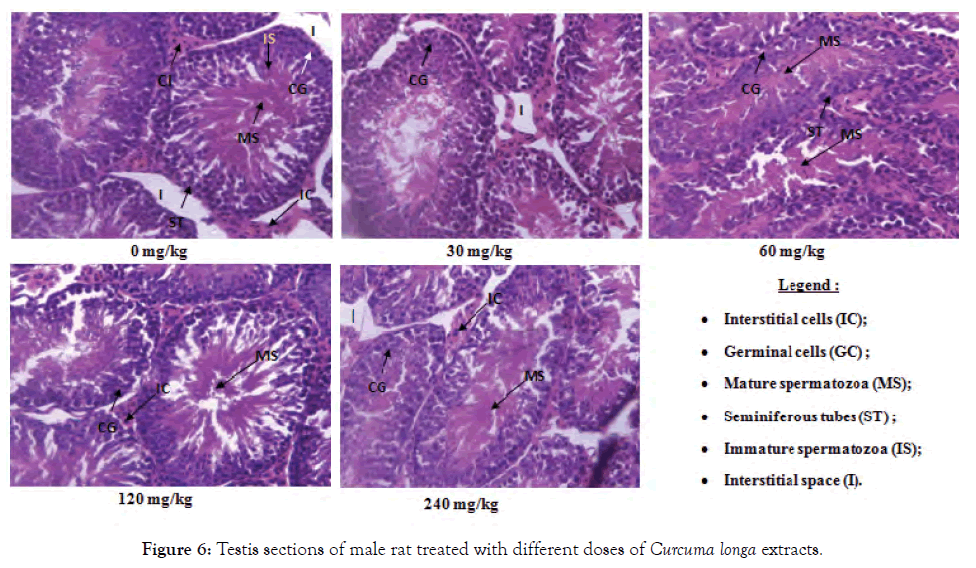
Figure 6: Testis sections of male rat treated with different doses of Curcuma longa extracts.
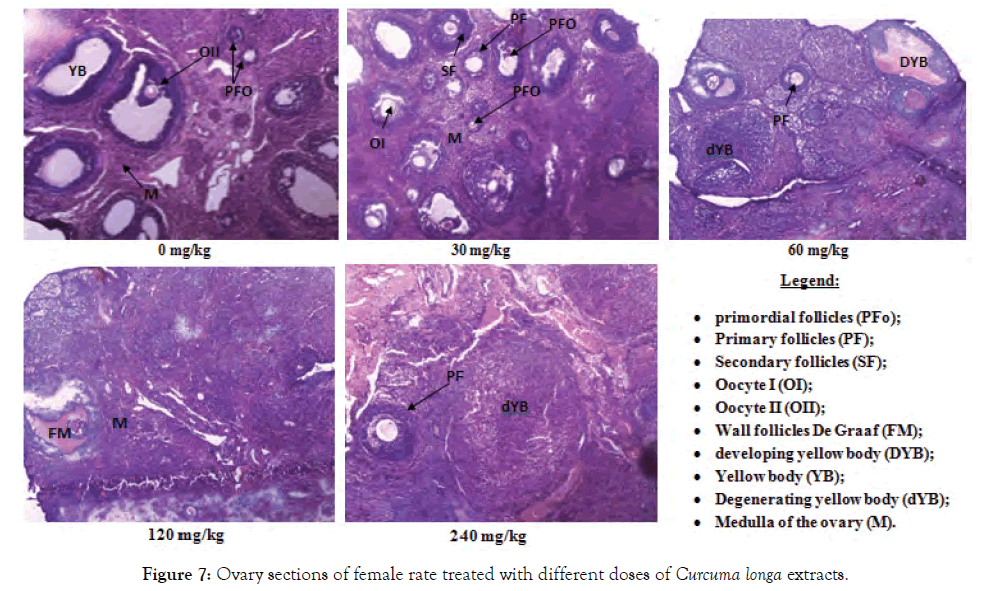
Figure 7: Ovary sections of female rate treated with different doses of Curcuma longa extracts.
Discussion
In vivo safety assessment remains the most useful tool for identifying target organ toxicity, despite advances in in vitro toxicity testing [12] because, the organism is a variable medium and could cause serious changes (positive or negative) in drug substances.
After single-dose administration of extracts, no deaths were recorded at the 5000 mg/kg limit dose; this shows that the LD50 is greater than 5000 mg/kg and therefore, the extract is almost non toxic or relatively harmless. This could be justified by the fact that C. longa is a food plant of the Zingiberaceae family whose safety is known for several plants of this family [13]. These results are in agreement with those of [14] which had shown an LD50 of this extract in mice above 15 g/kg body weight.
In sub-chronic study, both food consumption and weight gain were concomitant, which could imply a direct effect of dietary intake on weight loss or gain in all tested animals of both sexes. Daily administration of different doses of C. longa extract resulted in an increase in feed consumption at all doses compared to the control group. This result suggests that the ethanolic extract of C. longa could give the animals an appetite. This result corroborates those of Kodjio et al. [5] and Yassir [15], who had already shown that plant extracts could provide appetite to rats.
The liver is the central organ of metabolism. In cases of hepatic injury, there is an increase in transaminase activity (ALT, AST) and when the damage increases, this activity also increases [9]. However, the decrease in serum transaminase levels seen in the study compared to the control could reflect the hepatoprotective effect of the ethanol extract of C. longa in rats. These results are in agreement with those of Joshi et al. [16] who had shown that curcumin extracted from C. longa had a hepatoprotective effect. Indeed, curcumin is the major compound of this plant and could enhance the selective permeability of cell membranes; this will result in lower ALT and serum AST [16]. This decrease in serum transaminase is the result of the accumulation of these enzymes in the liver; consequence of the high level of liver protein observed. Just as the decline in serum protein observed in this work, confirm the hepatoprotective effect of the ethanolic extract that limit the degradation of hepatocytes by decreasing the amount of serum transaminase and other enzymes that must be released into the blood, because an increase in serum proteins could reflect a cellular lesion [17]. Similarly, the decrease in serum triglyceride levels observed in this work further confirms the protective effect of the extract on the liver because hepatotoxicity is generally associated with hypertriglyceridemia [16,18]. This hepatoprotective effect of the extract is confirmed by histopathological analysis of the liver, which showed no lesions at all doses in all animals. This work correlates with those of Kodjio [19], which showed that aqueous extracts and the methylene chloride mixture of this plant also had a hepatoprotective effect in rats as it corrected the lesions created by Salmonella Typhi infection.
The decrease in triglycerides, total cholesterol, LDL cholesterol and the increase in HDL cholesterol observed in this work shows that the extract could have a lipid-lowering effect. The extract is lipid-lowering when it lowers serum levels of total cholesterol, triglycerides, LDL and leads to an increase in HDL levels [20,21]. This result corroborates those of Samani and Farrokhi [22], who showed that Curcumin extracted from C. longa had a lipid-lowering effect. An increase in LDL cholesterol, triglycerides and a decrease in HDL cholesterol increase the atherogenic index and therefore the risk of cardiovascular disease [23,24]. The ethanolic extract of C. longa could thus prevent cardiovascular diseases since it lowers LDL cholesterol, triglycerides and increases the level of HDL cholesterol in the blood.
The kidneys are the main system of excretion of drugs as well as their different metabolites. Because of this, they are regularly subjected to more or less high doses of these often toxic substances which can damage it. Serum and urinary creatinine levels, as well as urinary protein levels are used to evaluate the functioning of the kidney [25]. However, the decrease in serum creatinine and the increase in urinary creatinine observed in the test groups compared to controls could reflect the good functioning of the kidneys and especially that of glomeruli. The malfunction of the kidney results in increased serum creatinine and decreased urination [26]. The drop in the urinary protein content observed in this work could reflect here the nephroprotective effect of this extract. These results are confirmed by the histological sections of the kidneys which showed no abnormalities, further confirming that extracts of C. longa have a nephroprotective effect. This corroborates the work of Kodjio [19], who showed that the aqueous extracts and the methylene chloride/methanol mixture of this plant remedied the renal damage caused by Salmonella Typhi infection and the work of Khorsandi and Orazizadeh [27], which showed that this plant had nephroprotective properties against paracetamol poisoning.
The increase in white blood cell count observed in this work suggests that C. longa extract may have an immunostimulatory effect. An immunostimulatory extract would lead to an increase in white blood cells [28]. This result is consolidated by the increase in the level of splenic proteins which could be due to an increase in immunoglobulin synthesis capable of defending the body and any foreign body occurring. Ashoka et al. [29] had previously shown that the administration of immunostimulatory extracts is generally accompanied by an increase in the level of splenic proteins.
The constant rate of red blood cells observed shows that the plant extract does not affect erythropoiesis. This could reflect again the good functioning of the kidneys, because the slightest damage would have influenced the synthesis of the erythropoietin hormone that stimulates the bone marrow to make red blood cells [30]. Also, this result observed with red blood cells could reveal that the extract could not induce hemolysis. This extract could exert hemoprotective effects because according to Zohra and Fawzia [31], a plant that does not cause haemolysis protects the membranes of red blood cells against active agents that could get into the blood and therefore keep the red blood cell count constant.
The absence of anomaly observed on the histological sections of the gonads (testis and ovaries) of rats treated with different doses of plant extract reflects the no variation in the relative weight of the organs of these animals at all doses; proving that the extract would probably have no adverse effect on the reproductive organs and therefore on fertility. According to Ghalamoun-Slaimi and Guichaoua [32], the absence of tubular light, germ cells and Sertoli cells as well as the presence of rare spermatogonia and spermatocytes marks an abnormality of the testis. Nevertheless, the absence of De Graaf follicle or mature follicle during the ovarian cycle indicates an anomaly in the ovary.
Conclusion
The ethanolic extract of Curcuma longa is relatively harmless by acute and Sub-chronic oral administration. Therefore, this extract does not present any risk of toxicity for consumers at all doses used in this work. Thus, the 30 mg/kg dose of this extract can be used for the safe treatment of typhoid fever.
Conflicts of Interest
All authors declare that they have no conflicts of interest.
Acknowledgement
The authors are grateful to Laboratory of Anatomy and Pathological Cytology of the University Hospital of Yaoundé (Cameroon) for the realization of the histopathological section. To Nguemnin Kamseu Patriciale and to Ekom Endeguele Steve for their technicals assistances.
REFERENCES
- Garcia RC, Louredo VF, Mattedi WC, Garcia RP. Ensaios Biologicos do Almeirao-roxo (Cichorium intybus L.) and Barbatimão (Stryphnodendron barbatian Martius) em ratas com menopausa cirúrgica. Revista Eletrônica de Farmácia. 2010;7:16-16.
- Rahman MA, Rana MS, Zaman MM, Uddin SA, Akter R. Antioxidant, antibacterial and cytotoxic activity of the methanol extract of Urtica crenulata. J Sci Res. 2010;2:169-177.
- Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: The Indian solid gold. Exp Med Biol. 2009;595:1-75.
- Kodjio N, Atsafack SS, Fodouop SP, Kuiate JR, Gatsing D. In vitro anti-salmonellal and antioxidant activities of extracts and fractions of Curcuma longa L. Rhizomes (Zingiberaceae). Int J Biochem Res Rev. 2016;11:1-14.
- Kodjio N, Atsafack SS, Njateng G, Sokoudjou JB, Kuiate JR, Gatsing D. Antioxidant effect of aqueous extract of Curcuma longa rhizomes (Zingiberaceae) in the typhoid fever induced in Wistar rats model. J Adv Med Pharm Sci. 2016;7:1-13.
- Fakam NLA. Etude des mécanismes d’action et essai de standardisation des extraits d’une plante médicinale à activité anti-typhique: Curcuma longa. Mémoire de Master of sciences. University of Dschang (Cameroon), Department of Biochemistry. 2017.
- Poonam CK, Kruti S, Raxita P. Investigation of phytochemical screening and antimicrobial activity of Curcuma longa. Int J Adv Res Biol Sci. 2017;4:153-163.
- OECD. OECD Guidelines for the testing of chemicals no: 425 Acute oral toxicity -dose adjustment method. OECD. 2008;1-29.
- Gatsing D, Aliyu R, Kuiate JR, Garba IH, Jaryum KH, Tedongmo N, et al. Toxicological evaluation of the aqueous extract of Allium sativum bulbs on laboratory mice and rats. Cameroon J Exp Biol. 2005;1:39-45.
- OECD. Repeated dose oral toxicity test method. In: OECD Guidelines for testing of chemicals, N°407. Organization for Economic Cooperation and Development, Paris, France. 2008.
- Venkataranganna MV, Gopumadhavan S, Sundaram R, Peer G, Mitra SK. Pharmacodynamics & toxicological profile of PartySmart, a herbal preparation for alcohol hangover in Wistar rats. Indian J Med Res. 2008;127:460.
- Fielden MR, Kolaja KL. The role of early in vivo toxicity testing in drug discovery toxicology. Expert Opin Drug Saf. 2008;7:107-110.
- Puangsomchit A, Bullangpoti V, Pluempanupat W. Toxicity of Alpinia galanga (zingiberaceae) rhizome extracts against Spodoptera litura (Lepidoptera: Noctuidae). Communications in Agricultural and Applied Biological Sciences. 2014;79:145-149.
- Sittisomwong, N, Leelasangaluk V, Chivapat S, Wangmad A, Ragsaman P, Chuntarachaya C. Acute and sub-chronic toxicity of turmeric. 1991;32:101-111.
- Yassir DKA. Study of the biological effect of Thymus vulgaris extracts on spermatogenesis in experimentally infected white mice Balb/c by Toxoplasma gondii. International Journal of Scientific & Engineering Research. 2014;5:1-9.
- Joshi D, Mittal DK, Shukla S, Srivastav SK, Dixit VA. Curcuma longa Linn. extract and curcumin protect CYP 2E1 enzymatic activity against mercuric chloride-induced hepatotoxicity and oxidative stress: A protective approach. Experimental and Toxicologic Pathology. 2017;69:373-382.
- Emerson FS, Shadara AC, Pud P. Toxic effects of crude extract of Plumumeae rosea (Rokta chitcakal). J Ethnopharmacol. 1993;100:37-39.
- Siouda W, Abdennour C. Can Urtica dioica supplementation attenuate mercury intoxication in Wistar rats?. Veterinary World. 2015;8:1458.
- Kodjio N. Etude des propriétés anti-salmonelles, antioxydantes, toxicologiques et formulation galénique des extraits de Curcuma longa L. Ph.D Thesis. University of Dschang (Cameroon), Department of Biochemistry. 2017.
- Rajendran R, Krishnakumar E. Hypolipidemic activity of chloroform extract of Mimosa pudica leaves. Avicenna J Med Biotech. 2010;2:215.
- Fernandes CE, Kuhn F, Scapinello J, Lazarotto M, Bohn A, Boligon AA, et al. Phytochemical profile, antioxidant and hypolipemiant potential of Ilex paraguariensis fruit extracts. Ind Crop Prod. 2016;81:139-146.
- Samani KG, Farrokhi E. Effects of cumin extract on oxLDL, paraoxanase 1 activity, FBS, total cholesterol, triglycerides, HDL-C, LDL-C, Apo A1, and Apo B in in the patients with hypercholesterolemia. Int J Health Sci. 2014;8:39.
- Benhamou JP, Johanes B, Neil M, Mario R, Rodes J. Hepatologie Clinique. (1st edn) Flammarion. 1993.
- Atsafack SS, Kuiate JR, Mouokeu RS, Mogtomo MLK, Tchinda, AT, De Dieu TJ, et al. Toxicological studies of stem bark extract from Schefflera barteri Harms (Araliaceae). BMC Complementary and Alternative Medicine. 2015;15:44.
- Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349:474-485.
- Schaffler A, Menche N. Anatomy, Physiology, Biology (2nd edn). Malome, SA. (Ed). 2004.
- Khorsandi L, Orazizadeh M. Protective effect of Curcuma longa extract on acetaminophen induced nephrotoxicity in mice. DARU. 2008;16:155-159.
- Hwang PA, Wu CH, Gau SY, Chien SY, Hwang DF. Antioxidant and immune-stimulating activities of hot-water extract from seaweed Sargassum hemiphyllum. J Mar Sci Tech. 2010;18:41-46.
- Ashoka SM, Shastry CS, Sridevi K, Gopkumar P. Stimulation of immune function activity of the extract of Heliotropium indicum leaves. Int J Pharmacol. 2009;7:1-10.
- Cloutier L, René A, Jutras A. The complete blood formula. Knowledge applied to nursing practice. Perspective infirmiere: revue officielle de l'Ordre des infirmieres et infirmiers du Quebec. 2014;11:28-32.
- Zohra M, Fawzia A. Hemolytic activity of different herbal extracts used in Algeria. Int J Pharm Sci Research. 2014;5:495-500.
- Ghalamoun-Slaimi R, Guichaoua MR. Testicular histology and meiotic studies in non-obstructive type sterility. Basic Clin Androl. 2006;16:135-142.
Citation: Gatsing D, Kamsu GT, Chegaing Fodouop SP, Tagne RS, Kodjio N, Nguelebek Fakam AL (2019) Evaluation of the acute and sub-chronic toxicity of the ethanolic extract of Curcuma longa (Zingiberaceae) in Wistar albino rats. Mod Chem Appl 7:267. doi: 10.35248/2329-6798.19.7.267
Copyright: © 2019 Gatsing D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


