PMC/PubMed Indexed Articles
Indexed In
- Online Access to Research in the Environment (OARE)
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Scimago
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 12, Issue 8
Histological Aberrations in Selected Organs of two Clariids Species obtained from Asa River, Nigeria
Ogundiran Mathew Akinloye*, Fawole Olubanjo Olatunde and Ajala Olumuyiwa OlasunmiboReceived: 10-May-2021 Published: 25-Aug-2021
Abstract
Gross impact of water pollution and water borne pollutants on aquatic organisms and man has become a great concern to public health in the recent time. Since fish gills are indirect contact with the environmental medium and are very sensitive to changes in water quality while fish liver serves as gate-keeper to the entire body of fish. Therefore, the aim of this study was to evaluate the effects of pollutants from the polluted Asa River on the gills and liver histology of Clarias buthupogon and Heterobranchus longifilis. The sample included a total of 55 individuals (28 for C. buthupogon and 27 for H. longifilis). There was a recorded moderate to intense alterations of gill tissues. The most notable alterations were congestion of blood vessels, desquamation, swelling of pillar cell, hyperplasia and hypertrophy of the gill epithelium. Epithelial lifting of the basement membrane was common in virtually all sampled fish species, while circulatory disturbances were slightly pronounced. The liver showed congestion of central vein, degeneration of hepatocyte, sinusoidal distortion, cellular inflammation and necrosis. However, all histological alterations recorded in the gill and liver tissues were most likely to be caused by increased concentrations of certain pollutants from industrial, domestic and agricultural premises. Furthermore, these results represent an additional reason to proceed with a detailed monitoring of the Asa River and the wildlife therein, for the sake of public health.
Keywords
Histo-architecture; Clarias buthupogon; Heterobranchus longifilis; Asa River; Histopathology
Introduction
Decline in the populations of important fish species have become a severe problem in many Nigerian rivers over the past few decades and the reasons for this remained unclear. Water pollution has been suspected to be possible contributors [1]. Asa River in Nigeria is a typical example of a river in which fish populations have declined over the past years [2]. The freshwater ecosystem is threatened by increasing levels of various pollutants originating from anthropogenic activities, urban, agricultural and industrial discharges. A considerable number of chemicals have already been released into the environment and persist in sediment, water and biota [3].
Anthropogenic activity has had a significant impact on the hydrologic regime, water and sediment quality of river Asa, main sources of pollution to this river are irrigation channels which are not maintained properly, sewage effluents, effluents from industrial premises, fish ponds and livestock farms and so on [2,3].
Histopathological biomarkers are valuable indicators of the harmful effects of pollutants and potential pathogens. These markers are intermediate biomarkers in terms of ecological importance, response time and level of biological organization, and as such are very suitable for the assessment of potentially harmful effects of various pollutants [4]. Histopathological evaluation is a sensitive tool in toxicant impact assessment to indicate the effects of toxicants on fish health and also allows for early warning signs of disease and injury in cells, tissues, or organs. Such structural changes in fish as biomarkers in various tissues in different species have also been studied by many researchers [5-8]. Therefore, this present study aimed at evaluating the histo-architectural alterations in gill and liver tissues of Clarias Buthupogon and Heterobranchus Longifilis obtained from Asa River, Nigeria.
Materials and Methods
Samples of Clarias buthupogon and Heterobranchus longifilis were collected separately from the downstream portion of Asa River using standard fishing device. The samples include a total number of 55 fish, selected from the pool of fish collection, 28 for Clarias buthupogon and 27 for Heterobranchus longifilis. They were transported in pre-treated plastic containers to laboratory for histological analysis. The samples were sacrificed and dissected immediately; the second left gill arch from the left side was excised and the gill tissue obtained was immediately fixed in 10% formaldehyde. Also, liver sample were obtained and fixed. After 24 hours the fixed tissues were taken for histological technique using the modified method of Bernet D, et al., [1]. Sections were made at 5-6 μm thickness and stained with Hematoxylin and Eosin (H&E), stained slides were examined under light microscope and photographed (Labomed). A qualitative histological assessment was done to identify histological alterations in the gill and liver tissues of the sampled fish population. These results were assessed and analyzed using a protocol developed by Bernet D, et al. [1} and Takashima F, et al. [9].
Results and Discussion
The gill structure of the two fish species sampled showed varying proportion of histological degradations ranging from hypertrophy, desquamation of lamellae epithelium, edema, swelling of pillar and chloride cells, disappearance or obliteration of secondary lamellae and cellular necrosis (Figures 1 and 2). In addition to these changes, lifting of secondary lamellae epithelium (desquamation) and a pronounced fusion of adjacent lamellae observed in almost all gill structure examined was probably as a result of the pollution load of the surrounding medium.
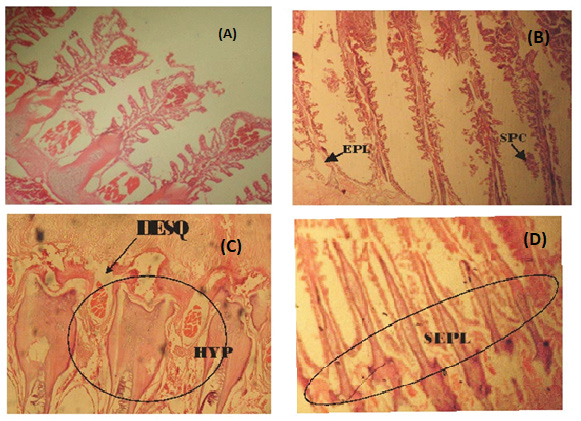
Figure 1: (A) Photomicrograph of a fairly normal gill tissue of C. buthupogon from a polluted portion of Asa River with mild congestion of blood vessels (B) Gill tissue of C. buthupogon from the polluted portion of Asa River showing severe epithelial lifting of secondary lamellae and Swelling of Pillar Cell (SPC) (C) Gill tissue of C. buthupogon from the polluted portion of Asa River with severe desquamation of secondary lamellae and hypertrophy (D) Gill tissue of C. buthupogon from the polluted portion of the river showing severe epithelial lifting of secondary lamellae (400x mg).
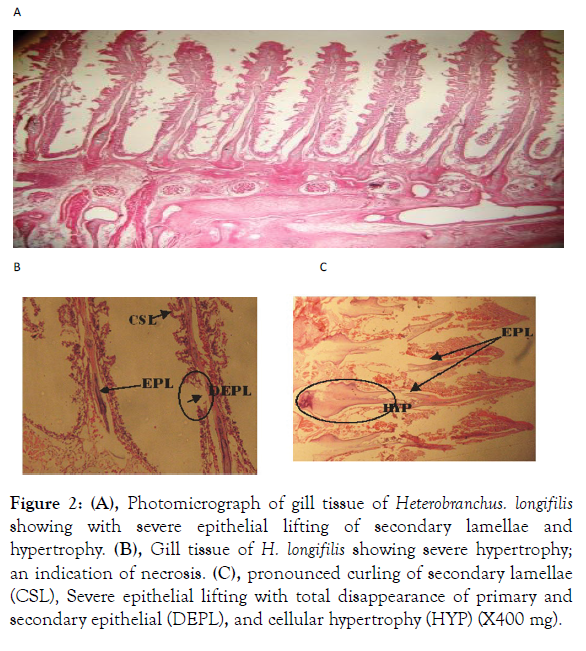
Figure 2: (A), Photomicrograph of gill tissue of Heterobranchus. longifilis showing with severe epithelial lifting of secondary lamellae and hypertrophy. (B), Gill tissue of H. longifilis showing severe hypertrophy; an indication of necrosis. (C), pronounced curling of secondary lamellae (CSL), Severe epithelial lifting with total disappearance of primary and secondary epithelial (DEPL), and cellular hypertrophy (HYP) (X400 mg).
The gill of a fish functions for respiration, ion regulation, acid – base balance and nitrogenous wastes excretion and constitute over 50% of the total surface area of the animal that make it sensitive to chemical changes in water. The reviews of Wood have provided extensive information on gill structural alterations in fish as a result of toxicants exposure. The edematous separation of the respiratory epithelium may be due to water borne toxins and this departure from normal structure affects the functional efficiency of the gills for gas transport regulation [10]. Also, the pathological changes in the pillar and chloride cells may be an indication of lamellae due to an obliteration of inter–lamellae space and necrosis of the respiratory epithelium followed osmoregulation dysfunction, which is the main function of these cells: Fusion of adjacent secondary lamellae on one or both sides of the primary by the degeneration of central blood sinuses.
Abnormal changes recorded in the gill tissue of the two investigated fish species, may also be an indication of the reaction of toxicants such as metal intake or adaptation to prevent the pollutants entry through the gill surface. Similar findings have been reported for other species that were exposed to different pollutants [6,11]. Likewise, Camargo and Martinez observed hypertrophy and hyperplasia of the epithelium cell, fusion of secondary epithelium, lifting of lamellae epithelium and congestion of blood vessels in the gills of P. lineatus being caged in Brazilian Cambe stream polluted by the industrial, domestic and agricultural wastes [12]. Triebskorn R, et al. [13] reported epithelial lifting, proliferation, necrosis and hyperplasia of mucous cells in gills of C. nasus and L. cephalus from River Merus in the Western Romania which was polluted by heavy metals, fecal coliforms and streptococci bacteria.
Several histological alterations were recorded in the gill tissues of the two fishes examined and this may be attributed either directly or indirectly to the impact of the river water pollution as fish gills are very sensitive to changes in the composition of their environment especially the water borne toxicants. Consequently, injury to the gill epithelium is a common response observed in the fish exposed to varieties of contaminants. Arellano JM, et al. [14] also observed a gross epithelial lifting of the secondary lamellae after exposure to heavy metals, such as cadmium and copper which agreed well with the present finding. Lifting of lamellae epithelium and lamellae fusion could be protective as it diminishes the extent of vulnerability of the gills surface area [15].
Generally, these types of alterations are associated with the presence of some toxic substances in the environment. All the histological changes observed in the gills of C. buthupogon and H. longifilis in this present work may have resulted from hypoxia, respiratory failure problems with ionic and acid base balance [16]. In the gills, only a few micrometers separated the blood from the water [17], which not only facilitate the exchange of gases, but also allow the gill tissue to be exposed to variations in the environment. Consequently the presence of toxic substances in the environment causes alterations in the vital functions carried out by the gills and alterations in the morphologic structure of these organs [18]. Thus, the histopathologic analysis of fish gills has been used as a tool that is extremely important in the evaluation of the quality of aquatic ecosystem. Fishes are intimately associated with their aqueous environment including physical and chemical changes in this ecosystem which reflect rapidly as quantifiable physiologic measurements in the fish.
In addition, reactions of fish gill to an irritant include inflammation, hyperplasia, lamellae fusion, over-secretion of mucus epithelial lifting, flattening and thickening of the secondary lamellae and so on. As a consequence of the epithelial lifting, there is an increase in the distance between the water and blood, impairing oxygen uptake. However, in this situation the fish increase their rate of respiration by compensating for the low entrance of oxygen [19]. According to Winkaler EU, et al. [12] these types of histopathologic lesions indicated that the fish responds to the effects of toxic agents present in the water and the sediment. Spaeare R, et al. [20] observed that alterations such as hyperplasia of the mucosal cells occur as a consequence of infection conditions or due to the presence of pollutants [20]. Winkaler et al., also observed severe histopathologic alterations such as lamellae aneurysm and epithelial lifting in specimens of Astyanax fasciatus and Astyanas altiparanae [12]. Similar results were obtained by authors such as Fernandes MN [19], Cerqueira CC, et al. [21], Oliveria-Ribeiro CA, et al. [22], Thophon S, et al. [23] and Stentiford GD, et al. [24].
Liver is vital organ that is most affected by the pollutants in the water due to its role in the detoxification and biotransformation processes. It also serves as an integrator for biochemical and physiological functions, and caries out key functions in excretion of xenobiotics. Histological alterations recorded in the liver of the studied species were in agreement with many studies that examined the effects of different pollutants on fish liver (Figures 3 and 4) [25,26]. They also observed degeneration of the hepatocytes and focal necrosis in the liver of C. gariepinus being exposed to be in consonance with the present study.
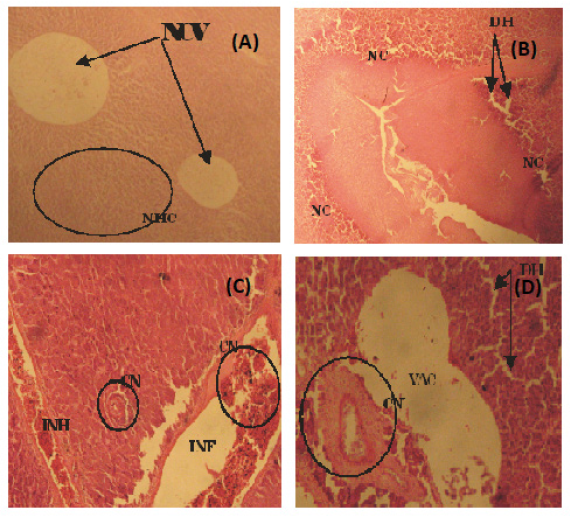
Figure 3: (A) Photomicrograph of a fairly normal liver tissue of C. Buthupogon from a polluted portion of Asa River revealing Normal Central Vein (NCV} and Hepatic Cells (HC) (B) Liver tissue of C. buthupogon showing Degenerated Hepatocytes (DH), sinusoidal distortion and Cellular Necrosis (NC) (C) Liver tissue of C. Buthupogon showing mild congestion of Central Vein (CN) with partial inflammation in between the Hepatocytes and Intravascular Haemolysis (INH) (D) Liver issue of C. Buthupogon showing severe Hepatic Degeneration (DH), vaculation or inflammation in-between hepatocytes with severe congestion of Central Vein (CN) (400x mg).
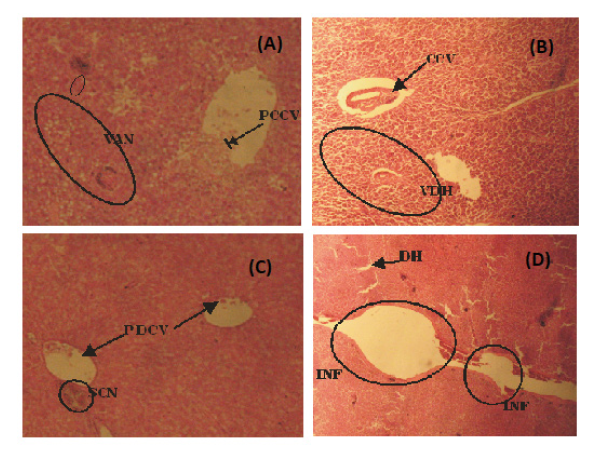
Figure 4: (A) Photomicrograph of liver tissue of H. longifilis from polluted portion of Asa River showing Partial Congestion of Central Vein (PCCV), Vocal Area of Cellular Necrosis (VAN) with sinusoidal distortion (B) Liver tissue of H. Longifilis showing Vacular Degeneration of Hepatocytes (VDH) and congested Central Vein (CN) (C) Liver tissue of H. longifilis showing Partial Congestion of Central Vein (PCCV) and a Severely Congested Area (SCN) (D) Liver tissue of H. longifilis showing severe Hepatic Degeneration (DH), vaculation of hepatocytes (400x mg).
Although, liver histological changes are not specific to pollutants, several studies have established a causal relationship. Varied degree of histological alterations was documented in this work; ranging from congestion of central vein, hepatocytes degeneration. Distortion of sinuses, inflammation, hemolysis, vacuolation and cellular necrosis: This finding is in conformity to several other findings world-wide [26]. The marked histological alterations observed in this study may be due to the additive or cumulative effects of increased metal concentrations in the liver. These results agreed with the findings of Authman MM, et al. [27], which submitted that the liver has an important detoxification role of endogenous wastes products as well as externally derived toxins such as heavy metals mixtures. The liver section of almost all the specimens examined in the work showed dilation and destruction of central vein. The hepatocytes revealed fatty degeneration with pronounced vacoulation and necrosis and manifestation of hemorrhage. Hemorrhagic lesions were abundant and this may be due to high pollution index or probably because of the inflammation of liver tissue in between hepatocytes.
Fatty degeneration of hepatocytes in the liver may also be attributed to an oxygen deficiency as a result of gill degeneration or the vacuolar dilation and intravascular hemolysis observed in the blood vessels [28]. Many authors have also reported similar histological alterations in fish livers that were exposed to metals [6,29]. Other studies revealed that vacoulation, inflammation and congestion were early stages in the hepatic degeneration, thus, these could be used as histological biomarker of different level of exposure [24,30]. Hepatic necrosis as generally observed in this work, has also been established to be the order of the day in fishes collected from contaminated ecosystem with metals [31-39]. The significantly high values of heavy metals in the liver could be linked to the occurrence of heterogeneous parenchyma in the liver of the two fish species in response to the metal of these fish to the polluted water of Asa River.
Therefore, it is possible to use gills and liver hepatocytotic alterations as a biomarker to assess the impact of heavy metals or other pollutants toxicity on fish health and production. This study however, investigated the non-suitability of fish from polluted sites with respect to anthropogenic discharge. Consequently, this study has been able to establish the fact that, exposure of fish to even low concentration of toxicant in the aquatic phase can induce various toxicological effects and histological degradations which depend on the period of exposure, physiological status of the resident species, volume of water (in terms of seasonal variation) and concentrations of such pollutants. Obtained results showed that the histological alterations in the gill and liver tissues are most likely caused by increased concentrations of certain pollutants. According to the previous water quality studies of the Asa River, metals concentrations were found at an elevated level. This information verifies that histopathological changes are valuable biomarkers for field evaluation, especially in tropical regions that are naturally affected by variety of environmental variations. It should be highlighted that histopathology is able to assess the initial effects and reactions to acute exposure to chemical stressors.
Conclusion
Conclusively, C. buthupogon and H. longifilisin Asa River are indeed responding to some stressors whose exact nature could be anthropogenic. According to the previous water quality studies of the Asa River, concentrations of iron, copper, cobalt, arsenic, lead, chromium and chlorine were at elevated levels and these substances could be responsible for the observed histopathological alterations. Present study represents an additional reason to proceed with a detailed monitoring of this river and the wildlife within it. Therefore, consumption of river foods from Asa River should be discouraged and urgent water monitoring system is required in the river.
Acknowledgement
We hereby acknowledge the technical and laboratory staffs of the Department of Pure and Applied Biology, Ladoke Akintola University of Technology, Ogbomoso, Nigeria for their kind support in terms of materials supplies.
REFERENCES
- Bernet D, Schmidt H, Meier W, Burkhardt‐Holm P, Wahli T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. J Fish Dis. 1999;22(1):25-34.
- Ayandiran TA, Fawole OO. Seasonal variation and length-weight relationship of Clarias gariepinus from Oluwa River, Nigeria. J Environ Sci Toxicol Food Technol. 2014;8(7):51-9.
- Akinloye OM, Olubanjo FO. Spatio-seasonal distribution and condition factors of Clarias buthupogon and Heterobranchus longifilis from Asa River, Nigeria. 2017.
- Van der Oost R, Beyer J, Vermeulen NP. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ Toxicol Pharmacol. 2003;13(2):57-149.
- Magar RS, Bias UE. Histopathological impact of malathion on the ovary of the freshwater fish Channa punctatus. Int Res J Environ Sci. 2013;2(3):59-61.
- Ogundiran MA, Fawole OO, Adewoye SO, Ay TA. Pathologic lesions in the gills of Clarias gariepinus exposed to sub-lethal concentrations of soap and detergent effluents. J Cell Anim Biol. 2009;3(5):078-82.
- McDonald DG, Wood CM. Branchial mechanisms of acclimation to metals in freshwater fish. Springer. 1993;p.297-321.
- Greco AM, Gilmour KM, Fenwick JC, Perry SF. The effects of soft water acclimation on respiratory gas transfer in the rainbow trout Oncorhynchus mykiss. J Exp Biol. 1995;198(12):2557-67.
- Takashima F, Hibiya T. An atlas of fish histology: Normal and pathological features.
- Kantham KP, Richards RH. Effect of buffers on the gill structure of common carp, Cyprinus carpio L., and rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis. 1995;18(5):411-23.
- Olurin KB, Olojo EA, Mbaka GO, Akindele AT. Histopathological responses of the gill and liver tissues of Clarias gariepinus fingerlings to herbicide-glyphosate. Afr J Biotechnol. 2006;5(24):2480.
- Winkaler EU, das Graças Silva A, Galindo HC, dos Reis Martinez CB. Histological and physiological biomarkers for monitoring the health of fish from streams of Londrina, State of Paraná. Acta Sci Biol Sci. 2001;23:507-14.
- Triebskorn R, Telcean I, Casper H, Farkas A, Sandu C, Stan G, et al. Monitoring pollution in River Mureş, Romania, part II: Metal accumulation and histopathology in fish. Environ Monit Assess. 2008;141(1-3):177-88.
- Arellano JM, Blasco J, Ortiz JB, Capeta-Da Silva D, Navarro A, Sanchez-Del Pino MJ, et al. Accumulation and histopathological effects of copper in gills and liver of Senegales Sole, Solea senegalensis and toad fish, Halobatrachus didactylus. Ecotoxicol Environ Restor. 2000;3(1):22-8.
- van Heerden D, Vosloo A, Nikinmaa M. Effects of short-term copper exposure on gill structure, Metallothionein and Hypoxia-inducible factor-1α (HIF-1α) levels in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol. 2004;69(3):271-80.
- Allen RG, Smith M, Perrier A, Pereira LS. An update for the definition of reference evapotranspiration. ICID bulletin. 1994;43(2):1-34.
- Wood CM, Soivio A. Environmental effects on gill function: an introduction. Physiol Zool. 1991;64(1):1-3.
- Poleksić V, Mitrović-Tutundžić V. Fish gills as a monitor of sub-lethal and chronic effects of pollution: Sub-lethal and chronic effects of pollutants on freshwater fish. 1994;339-52.
- Fernandes MN. Environmental pollution and fish gill morphology. Fish Adapt. 2003;203-31.
- Speare R, Freeland WJ, Bolton SJ. A possible iridovirus in erythrocytes of Bufo marinus in Costa Rica. J Wildl Dis. 1991;27(3):457-62.
- Cerqueira CC, Fernandes MN. Gill tissue recovery after copper exposure and blood parameter responses in the tropical fish Prochilodus scrofa. Ecotoxicol Environ Saf. 2002;52(2):83-91.
- De Oliveira Ribeiro CA, Belger L, Pelletier E, Rouleau C. Histopathological evidence of inorganic mercury and methyl mercury toxicity in the arctic charr (Salvelinus alpinus). Environ Res. 2002;90(3):217-25.
- Thophon S, Kruatrachue M, Upatham ES, Pokethitiyook P, Sahaphong S, Jaritkhuan S. Histopathological alterations of white seabass, Lates calcarifer, in acute and sub-chronic cadmium exposure. Environ Pollut. 2003;121(3):307-20.
- Stentiford GD, Longshaw M, Lyons BP, Jones G, Green M, Feist SW. Histopathological biomarkers in estuarine fish species for the assessment of biological effects of contaminants. Marine Environ Res. 2003;55(2):137-59.
- Ptashynski MD, Pedlar RM, Evans RE, Baron CL, Klaverkamp JF. Toxicology of dietary nickel in Lake Whitefish (Coregonus clupeaformis). Aquat Toxicol. 2002;58(3-4):229-47.
- Fanta E, Rios FS, Romão S, Vianna AC, Freiberger S. Histopathology of the fish Corydoras paleatus contaminated with sub-lethal levels of organophosphorus in water and food. Ecotoxicol Environ Saf. 2003;54(2):119-30.
- Authman MM, Abbas HH. Accumulation and distribution of Copper and Zinc in both water and some vital tissues of two fish species (Tilapia zillii and Mugil cephalus) of Lake Qarun, Fayoum Province, Egypt. Pakistan J BiolSci. 2007;10(13):2106-22.
- Mohamed FA. Impacts of environmental pollution in the southern region of Lake Manzalah, Egypt, on the histological structures of the liver and intestine of Oreochromis niloticus and Tilapia zillii. J Egyptian Acad Soci Environ Dev. 2001;2(2):25-42.
- Ogundiran MA, Fawole OO, Adewoye SO, Ayandiran TA. Toxicological impact of detergent effluent on juvenile of African Catfish (Clarias gariepinus) (Buchell 1822). Agri Biol J North Am. 2010;1(3):330-42.
- Au DW. The application of histocytopathological biomarkers in marine pollution monitoring: A review. Marine pollution bulletin. 2004;48(9-10):817-34.
- Olojo EA, Olurin KB, Mbaka G, Oluwemimo AD. Histopathology of the gill and liver tissues of the African catfish Clarias gariepinus exposed to lead. Afr J Biotechnol. 2005;4(1):117-22.
- Gurr E. Methods of analytical histology and histochemistry. Leonard Hill. 1958.
- Jebali J, Banni M, Boussetta H. Biochemical biomarkers in aquatic ecotoxicology: Fundamental mechanisms, application and perspectives. Adv Environ Res. 2012;23:143-68.
- Mazon AF, Monteiro EA, Pinheiro GH, Fernadez MN. Haematological and physiological changes induced by short-term exposure to copper in the freshwater fish, Prochilodus scrofa. Braz J Biol. 2002;62:621-31.
- Reddy PB. Histopathogical studies as potential and direct biomarkers of pollution. Trends Life Sci. 2012;1(1):27-31.
- Reddy PB, Baghel BS, Kushwah A, Ishaque S. Biochemical and haematological studies on effect of textile industry effluent in Muscus albinus. ICEM. 2010;pp:713-719.
- Rosety-Rodrı́guez M, Ordoñez FJ, Rosety M, Rosety JM, Rosety I, Ribelles A, et al. Morphohistochemical changes in the gills of turbot (Scophthalmus maximus L) induced by sodium dodecyl sulfate. Ecotoxicol Environ Saf. 2002;51(3):223-8.
- Viarengo A, Lowe D, Bolognesi C, Fabbri E, Koehler A. The use of biomarkers in biomonitoring: A two-tier approach assessing the level of pollutant-induced stress syndrome in sentinel organisms. Comp Biochem Physiol C Toxicol Pharmacol. 2007;146(3):281-300.
- Wood CM. Toxic responses of the gill: In Target organ toxicity in marine and freshwater teleosts. 2017;pp:1-89.
Citation: Ogundiran MA, Fawole OO, Ajala OO (2021) Histological Aberrations in Selected Organs of two Clariids Species obtained from Asa River, Nigeria. J Aquac Res Development. 12:651.
Copyright: © 2021 Ogundiran MA, et al. This is an open access article distributed under the term of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.