Indexed In
- Open J Gate
- Genamics JournalSeek
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 10, Issue 5
Evaluation of Antidiabetic and Antioxidant Potential of Hydromethanolic Seed Extract of Datura Stramonium in Mice
Bamlaku Cherie Melaku and Gedefaw Getnet*Received: 09-Apr-2020 Published: 17-Jul-2020
Abstract
Background: Datura Stramonium Linn is one of the folkloric medicines in Ethiopia and demonstrated in vitro antidiabetic activity. Thus, the current study was carried out to evaluate antidiabetic activity of hydromethanolic seed extract in mice.
Methods: The seed of Datura Stramonium was extract by using hydromethanol. The effect of the seed extract on blood glucose level was assessed after oral administration of 100, 200 and 400 mg/kg doses in normal, oral glucose loaded and Streptozocininduced diabetic mice.
Antioxidant capacity of seed extract was evaluated by using a 2, 2-diphenyl-1-picrylhydrazyl assay.
Results: Hypoglycemic effect of all doses of seed extract was insignificant (p>0.05) in normoglycemic model but glucose reduction was significant (p<0.05 at 100 mg/kg, p<0.01 at 200 mg/kg and 400 mg/kg) with respect to negative control in oral glucose loaded mice. All doses of seed extract significantly (p<0.0l) reduced blood glucose level on day 7 and 14 in STZ induced daily treated diabetic mice compared to the negative control. At the same time, 200 and 400 mg/kg doses of seed extract significantly (p<0.05) improved body weight of diabetic mice on day 7 and 14 but 100 mg/kg dose was delayed and significantly (p<0.05) increased body weight of mice on day 14 compared to the vehicle. The finding showed that antioxidant capacity of the seed extract was concentration dependent and comparable with ascorbic acid. IC50 of the seed extract and ascorbic was found to be 11.95 and 5.07 mg/mL, respectively.
Conclusion: The finding of the study showed that hydromethanolic seed extract of Datura Stramonium Linn endowed significant antihyperglycemic and scavenging of free radicals.
Keywords
Diabetes, Streptozotocin, Datura Stramonium, antioxidant, Mice
Abbreviations
GLUT2: Glucose transporter 2; GLUS- 4: Glucose transporter 4; OECD: Organization for Economic Cooperation and Development; STZ: Streptozocin; BGL: Blood glucose level; BW: Body weight
Introduction
Diabetes mellitus is chronic metabolic disorder characterized by hyperglycemia due to impaired insulin secretion or action or both. Uncontrol hyperglycemia cause suffering of the patients with life threatening complications (retinopathy, nephropathy, and peripheral neuropathy and acquired cardiovascular diseases)[1-5]. The prevalence and the burden of diabetes mellitus is very high in the world. In 2017, 425 million people (aged 20–79 years) live with diabetes and this number will rise to 629 million in 2045 globally [6]. The management of diabetes mellitus is challenging due to deleterious effects and limited efficacy of currently available antidiabetic drugs [7-9]. Medicinal plants with documented traditional uses are source of noble antidiabetic drugs and metformin (N, N dimethyl biguanide) is preferable antidiabetic drug derived from useful medicinal plant, Galega officinalis [10,11]. More than 800 plants have been used in the treatment of diabetes [12]. In recent years, catechol glycoside esters, isolated from the leaves of Dodecadenia grandiflora showed comparable antidiabetic effect with metformin and protodioscin isolated from seed of fenugreek demonstrated significant blood glucose lowering effect in rats and human [12,13].
Datura Stramonium Linn (Astenagra in Amharic). is one of the wellknown medicinal plant that belonging to the genus Datura and the family Solanaceae and originates in Americas. But now a day the plant founds around the world, including the warmer regions of North, Central and South America, Europe, Asia, and Africa and distributed throughout the Ethiopia [14,15]. The plant is a widespread annual plant and grows to 1.2 m high and the root is long, thick, fibrous, white and the stem is stout, erect, leafy, smooth, and pale yellow-green (Figure 1). The stem forks off repeatedly into branches and each fork forms a leaf and a single, erect flower. Alanine, glutamate, phenylalanine, tyrosine (amino acids) and antimuscarinic drugs such as scopolamine, atropine and hyoscyamine were isolated from seeds of Datura Stramonium [16-19].

Figure 1: Photograph of Datura stramonium Linn.
Datura Stramonium Linn used to treat diabetes mellitus, asthma, gastrointestinal problems, wounds, inflammation and tumors traditionally [16,20,21]. Experimental studies revealed that Datura stramonium possesses antiepileptic, anti-obesity, anti-microbial, antiviral, anticholinergic and bronchodilator activities [16,18,21,22]. The leaf and root extracts of Datura Stramonium exhibited free radical scavenging and anti-inflammatory activities [21,23]. Hydromethanolic root extract of the plant demonstrated significant in vivo antidiabetic and antidyslipidemic activity [24-26]. More importantly, the seed extract of Datura Stramonium demonstrated strong in vitro α-glycosidase and α-amylase inhibitory effect despite lack in vivo investigated for its potential antidiabetic activity. These call initiation to evaluate antidiabetic activities of hydromethanolic seed extract of Datura Stramonium in vivo.
Methods
Drugs, chemicals and instruments
The following drugs, chemicals and equipments were used in this experimental study.
Drugs: Streptozotocin (Sigma Aldrich, Germany), glibeclamide, (Julphar Pharmaceuticals),
Chemicals: 80% methanol, FeCl3 (MA, USA), NaOH (India), HCl (Suppertek Chemical), 40% glucose solution (Shandong, China),
Equipments: Lyophilizer, i-QARE DS-W® blood glucose meter, and test strips (Alliance International, New Taipei City, Taiwan), scissors, mask, animal cages, insulin syringe with needle, oven, desiccators.
Plant materials collection
The seeds of Datura Stramonium Linn were collected from the Wollo in October, 2019. Plant identification was carried out and the specimen of the plant material was deposited in the herbarium of biology department for future reference in Wollo University with a voucher number GG-004/2019.
Preparation of crude extract
The Seeds were wash with distilled water, dried under shade and then dried seeds were reduced into coarse powder by using electric mill. About 200 gram coarse powder of the seed was maceration in hydromethanol for seventy-two hrs. Hydromethanol with dissolved plant material was filtered by Whatman filter paper No.1. The marc was re-macerated two times in 80% methanol for seventy-two hours and the filtrates were concentrated by using rotary evaporator and dried in an oven at 40°C and lyophilizer was used to remove water with 24 hr.
Finally, collected seed extract was kept by using vial and stored in desiccator until used for the experiment.
Experimental animals
The mice were obtained from animal house of pharmacology department, Wollo university and were kept in 12 hrs light 12 hrs dark cycle with pellet diet and water ad libitum. Healthy male Swiss albino mice with body weight of 20 gram-35 gram and age of 8-12 weeks were used in the experimental study and healthy female mice with the same weight and age were used for acute oral toxicity study. This study was carried out based on the guide for the care and use of laboratory animals [27].
Phytomolecules evaluation of leaf extract of D. stramonium Qualitative preliminary screening was performed for seed extract by using standard reagent and procedures [28,29].
Toxicity study
Acute oral toxicity study was conducted according to OECD guideline 425 [30]. One healthy female mouse was fasted for 3-4 hr and then, body weight was measured. Then, 2000 mg/kg 80% methanolic seed extract was loaded orally to the fasted mouse and then, the mouse was strictly observed for 24 hr of physical and behavioral changes, given special attention to the first four hr. Based on the result from the first mouse, other four mice were fasted for 3-4 hr and fasted body weight was measured. 2000 mg/kg of 80% methanolic seed extract was loaded orally to each fasted mouse and were observed for 24 hr to determine physical and behavioral changes of mice. The follow up was continued for fourteen days for any physical and behavioral changes of the mice before initiation of the experiment.
In vitro antioxidant activity of the hydromethanolic seed extract of D. stramonium
Antioxidant capacity of the seed extract was evaluated in 2, 2-diphenyl-1-picrylhydrazyl method [30]. 3.9 ml of solution of DPPH (4 mg DPPH/100 ml methanol) was mixed with a 0.1 ml methanolic solution of different concentrations (5, 10, 20, 40, and 80 mg/mL) extract and incubated in the dark for half hour.
Ascorbic acid used as standard antioxidant. After half hour, the absorbance of the mixture and the control at 517 nm were read by using a UV spectrophotometer. The test was done in triplicate and the percent of scavenging of inhibition of was calculated as:
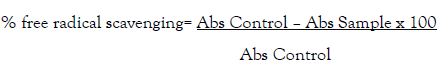
Where Abs Control was the absorbance without sample, Abs samples was the absorbance of sample latex or ascorbic acid.
Streptozocin induced experimental diabetes
Streptozocin (STZ) was dissolved in 0.1 M fresh cold citrate buffer (pH 4.5). Experimental diabetes was induced on overnight fasted (16 hr.) mice by i.p. injection of fresh solution of Streptozocin at the dose of 150 mg/kg [31-33]. After thirty minutes of Streptozocin injection, free access to food was allowed to the animals. For the next 24 hr after STZ injection, excessive insulin release from partially raptured pancreatic beta cells leads to hypoglycemic shock and death. To prevent this, after six hours of STZ injection, 5% glucose solution was given to each mouse for one day. After three days of STZ administration, fasting blood glucose level (BGL) of the mice >200 mg/dl were diabetic and recruited in the study [34-36].
Measurement of blood glucose level
Fasted blood glucose level of each mouse was measured in triplicate via a glucometer and strip by cutting the tail and the average value was taken.
Evaluation of hypoglycemic effect of seed extract in normoglycemic mice
The normal male mice were fasted overnight (16 hr) and d randomly grouped into five groups (n=6). Group I (negative control) was treated with 10 ml/kg distilled water and Group II (positive control) was treated 5 mg/kg glibeclamide, Group III, IV and V were treated with 100 mg/kg, 200 mg/kg and 400 mg/kg seed extract, respectively. Fasted BGL of each mouse was measured at 0 hour (before treatment) and at 1, 2, 4 and 6 hr post treatment.
Evaluation of the effect of seed extract on blood glucose level in oral glucose loaded mice
Sixteen hours (16 hr) fasted male mice were used to evaluate antihyperglycemic effect of the extract after oral glucose administration [37-42]. Normoglycemic overnight fasted mice were randomly grouped into 5 (n=6). Group I and II (negative and positive control) were treated with 10 ml/kg distilled water and 5 mg/kg glibeclamide, respectively; Group III, IV and V were treated with 80% methanolic seed extract at the dose of 100 mg/kg, 200 mg/kg and 400 mg/kg, respectively. Then, after thirty minutes, oral 2 g/kg of 40% glucose solution was administered to each mouse [33,40]. BGL of the mice were measured just before glucose administering and then at 0.5 hr, 1 hr and 2 hr after glucose administering [36,43,44].
Evaluation of the effect of seed extract on blood glucose level and body weight in STZ induced diabetic mice
Overnight (16 hr) fasted STZ induced diabetic mice were randomly grouped into 6 (n=6). Group I was treated with 10 ml/kg distilled water; Group II (diabetic positive control) was treated with 5 mg/ kg glibeclamide; Group III, IV and IV (diabetic test groups) were treated with 80% methanolic seed extract at the dose of 100 mg/ kg, 200 mg/kg and 400 mg/kg once daily for 14 days, respectively. After three days of STZ injection, fasting BGL and body weight of each mouse was measured. Then, fasting BGL and weight of daily treated each normal and diabetic mouse was measured two hours after treatment on day 7 and 14 [40,45].
Statistical analysis
Data with a group and b/n the groups were compared by using oneway ANOVA followed by Tuckey’s post hoc multiple comparison test. P-values <0.05 were considered statistically significant and SPSS Version 23 Software was used for statistical analysis.
Results
Acute oral toxicity test
Sign of toxicity and mortality associated with 2000 mg/kg loading hydromethanol seed extract of D. stramonium not exhibited on fasted female mice. Therefore, LD50 of hydromethanolic leaf extract is >2000 mg/kg. The three doses (100 mg/kg, 200 mg/kg and 400 mg/kg) of the leaf extract were determined based on acute toxicity result for experimental studies.
Phytochemical screening of hydromethanolic seed extract of D. stramonium
Qualitative preliminary phytochemical study showed that hydromethanolic seed extract contained saponins, alkaloids, flavonoids, phenols, tannins, terpenoids, glycosides and steroids (Table 1).
Table 1: Phytochemical screening of 80% methanolic seed extract of D. stramonium.
| Phytoconstituents | Result | |
|---|---|---|
| Flavonoids | + | |
| Phenols | + | |
| Tannins | + | |
| Saponins | + | |
| Alkaloids | + | |
| Terpenoids | + | |
| Glycosides | + | |
| Steroids | + | |
| Anthraquinones | - | |
Key: +: present, –: absent
Antioxidant activity of the seed extract of D. stramonium The finding of the study showed that free radical scavenging activity of the seed extract was concentration dependent and comparable to ascorbic acid. IC50 of the seed extract and ascorbic acid in the assay was found to be 11.95 and 5.07 mg/mL, respectively (Table 2).
Table 2: Percentage of free radical scavenging activity of the seed extract of D. stramonium.
| Concentration(mg/mL) | % of DPPH Inhibition | IC50 | ||
|---|---|---|---|---|
| AA | SE | AA | SE | |
| 5 | 28.22 ± 0.21 | 6.67 ± 0.71 | ||
| 10 | 45.66 ± 0.42 | 14.14 ± 0.53 | 4.97 | 11.95 |
| 20 | 66.81 ± 0.42 | 21.01 ± 0.41 | ||
| 40 | 80.26 ± 0.45 | 28.01 ± 0.31 | ||
| 80 | 92.53 ± 0.54 | 40.54 ± 0.27 | ||
Note: Values of % inhibition of DPPH free radical is described as Mean ± standard error of the Mean. DPPH-2,2-diphenyl-1-picrylhydrazine, AA-Ascorbic acid; SE-seed extract, IC-Inhibitory concertation
Effect of hydromethanolic seed extract of D. stramonium in normoglycemic mice
All doses of hydromethanolic seed extract of D. stramonium was produced insignificant (p>0.05) hypoglycemic effect at all time points but standard drug (5 mg/kg glibeclamide) showed significant (p<0.001) hypoglycemic effect at 2, 4 and 6 hr with respect to 100 mg/kg dose and negative control (Table 3).
Table 3: Effect of hydromethanolic seed extract of D. stramonium in normoglycemic mice.
| Fasting BGL (mg/dl) | |||||
|---|---|---|---|---|---|
| Groups | 0hr | 1hr | 2hr | 4hr | 6hr |
| 10 ml/kg NC | 90.46 ± 0.31 | 88.29 ± 0.75 | 80.52 ± 0.68 | 75.82 ± 1.71 | 75.06 ± 2.03 |
| 5 mg/kg GB | 89.28 ± 1.02 | 72.82 ± 0.91 | 66.79 ± 1.06a3b2 | 60.39 ± 2.29 a2b1 | 56.45 ± 2.09 a2b1 |
| 100 mg/kg SE | 90.53 ± 1.75 | 87.21 ± 0.42 | 79.58 ± 0.74c1 | 74.83 ± 1.73 c1 | 74.09 ± 0.39 c1 |
| 200 mg/kg SE | 89.53 ± 0.45 | 86.45 ± 1.90 | 77.88 ± 1.30 | 73.97 ± 1.17 | 73.56 ± 2.03 |
| 400 mg/kg SE | 90.06 ± 1.64 | 85.51 ± 0.46 | 77.62 ± 1.29 | 72.88 ± 1.25 | 72.11 ± 2.69 |
Key: Each data describes as mean ± standard error of the mean, n=6, acompared to negative control; bto 100mg/kg, cto 5mg/kg GB, 1 P<0.05; 2 P<0.001. BGL-blood glucose level; NC-negative control; GB-glibeclamide; SE-seed extract
Effect of hydromethanolic seed extract of D. stramonium on blood glucose level of oral glucose loaded mice
There was no significant variation in fasting blood glucose level the mice before oral glucose administration in all groups (Figure 2). The maximum blood glucose level was measured thirty minutes after oral glucose administration in all groups. BGL reduction of the seed extract was significantly (p<0.05 at 100 mg/kg, (p<0.01, at 200 mg/kg and 400 mg/kg) after 60 and 120 minutes oral glucose administration compared to the negative control. Blood glucose level reduction of 100 mg.kg dose was significantly (p<0.05) lower than 5 mg/kg dose glibeclamide (p<0.001, after 60 and 120 minutes).
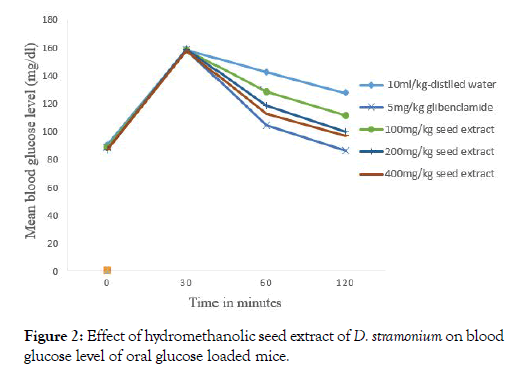
Figure 2: Effect of hydromethanolic seed extract of D. stramonium on blood glucose level of oral glucose loaded mice.
Antihyperglycemic effects of hydromethanolic seed extract of D. stramoniumin diabetic mice
After induction of diabetes, fasted blood glucose levels of daily treated diabetic mice were measured once weekly. The seed extract at the doses of 100 mg/kg, 200 mg/kg and 400 mg/kg significantly (p<0.0l) reduced fasted BGL on day seven and fourteen of daily treated diabetic mice compared to the control (Table 4).
Table 4: Antihyperglycemic effects of 80% methanolic seed extract of D. stramonium in diabetic mice.
| Group | Fasting BGL (mg/dl) | ||
|---|---|---|---|
| Day 0 | Day 7 | Day 14 | |
| 10ml/kg NC | 254.62±1.05 | 266.00±1.73 | 268.94 ± 0.49 |
| 5mg/kg GB | 255.46±0.34 | 243.22±0.42a2 | 240.18 ± 1.63 a2 |
| 100mg/kg SE | 256.06±1.27 | 255.06±0.57a1 | 250.22 ± 0.29a1 |
| 200mg/kg SE | 255.44±0.88 | 249.17±1.06a1 | 248.55 ± 1.06a1 |
| 400mg/kg SE | 255.89±0.92 | 246.45±0.51a1 | 247.17 ± 0.25a1 |
Key: Each data describes as mean ± standard error of the mean, n=6, acompared to negative control; 1P<0.01; 2P<0.001; BGL-blood glucose level; NC-negative control; GB-glibeclamide; SE-seed extract
Antihyperglycemic effect of glibeclamide was significant (p<0.001) on day seven and fourteen compared to the diabetic control. There was no significant BGL variation among seed extract and standard drug in STZ induced model.
Effect of hydromethanolic seed extract of D. stramonium on body weight of diabetic mice
The seed extract at the doses of 200 mg/kg and 400 mg/kg significantly (p<0.05) increased BW of diabetic mice on day 7 and 14 compared to diabetic control (Table 5). Body weight improvement of 100 mg/kg dose was delayed and significantly (p<0.05) day 14 compared to the negative control. In addition, glibeclamide significantly (p<0.01) improved body weight of diabetic mice on day 7 and 14 with respect to negative control.
Table 5: Effect of 80%methanolic seed extract of D. stramonium on body weight of diabetic mice.
| Groups | Body weight (g) | ||
|---|---|---|---|
| Day 0 | Day 7 | Day 14 | |
| 10 ml/kg NC | 22.89 ± 0.69 | 22.84 ± 0.51 | 23.08 ± 0.64 |
| 5 mg/kg GB | 23.43 ± 0.33a2 | 27.59 ± 0.31a2 | 28.26 ± 0.46a2 |
| 100 mg/kg SE | 22.59 ± 0.55a | 24.59 ± 0.64a1 | 25.48 ± 0.49a1 |
| 200 mg/kg SE | 23.87 ± 0.23a | 26.34 ± 0.53a1 | 25.91 ± 0.61a1 |
| 400 mg/kg SE | 24.27 ± 0.63a | 27.21 ± 0.39a1 | 26.72 ± 0.34a1 |
Key: Each data describes as mean ± standard error of the mean, n=6, acompared to negative control; 1 P<0.05; 2 P<0.01; BGL-blood glucose level; NC-negative control; GB-glibeclamide; SE-seed extract
Discussion
Datura Stramonium has been used in Ethiopian folklore medicine and demonstrated in vitro blood glucose reduction activity by inhibiting α-glucosidase and α-amylase without in vivo test. Therefore, invivo blood glucose lowering activity of hydromethanolic seed extract of D. stramonium was experimentally evaluated by using normoglycemic, oral glucose loaded and Streptozocin induced diabetic in mice. Hydromethanol (80% methanol) was selected as solvent of the extraction since a wide verity of polar and moderately polar phytomolecules extracted in hydromethanol [26,35,46].
The finding of this study showed that all doses of hydromethanolic seed extract were devoid significant hypoglycemic effect at all time points compared to negative control but glucose reduction of standard drug (5 mg/kg glibeclamide) was significant (p<0.01) at 2, 4, and 6 hr. Thus, mechanism of action of glucose lowering activity of seed extract and glibeclamide might not be the same. Similar to this finding, hydromethanolic seed extract of Calpurnia aurea [47] and root extract of D. stramonium [26] devoid significant hypoglycemic activity. In contrast, hydromethanolic leaf extract of Caylusea abyssinica, [35] leaf latex of Aloe vera [48] and Aloe megalacantha [49] demonstrated significant blood glucose lowering activity in normoglycemic model.
In glucose tolerance test, blood glucose reduction of the hydromethanolic seed extract was significantly (p<0.05 at 100 mg/ kg, (p<0.01, at 200 mg/kg and 400 mg/kg) after 60 and 120 minutes oral glucose administration compared to the negative control. Blood glucose reduction of 100 mg/kg dose was significantly (p<0.05) lower than 5 mg/kg dose glibeclamide (p<0.001, after 60- and 120-minutes). Similar to this finding hydromethanolic seed extract of Calpurnia aurea, [47] leaf extract of Caylusea abyssinica, [35] root extract of D. stramonium, [26] leaf extract of M. stenopetala [45] and Aloe megalacantha [49] have been demonstrated a significant glucose reduction in oral glucose loaded mice.
In STZ induced diabetic mice treated with 100 mg/kg, 200 mg/kg and 400 mg/kg doses of the hydromethanolic seed extract showed significant (p<0.01) glucose level reduction on day 7 and 14 with respect to diabetic control. This showed that the plant endowed antidiabetic activity and in line with administration of the same dose hydromethanolic seed extract of Calpurnia aurea, [47] root extract of D. stramonium, [26] leaf extract of M. stenopetala, [45] leaf latex of Aloe megalacantha [49] in streptozotocin-induced diabetic model.2 In another study, administration of leaf extract of A. vera at doses of 200 and 400 mg/kg showed comparable glucose reduction with 50 mg/kg metformin [50]. Seed extract of Datura metel in the genus Datura, has been showed to have significant blood glucose lowering activity [51].
At the same time, there was significant improvement of body weight in seed extract and standard drug treated groups in respect to diabetic control. Body weight of the mice treated with all doses of the hydromethanolic seed extract significantly improved on day 7 and 14. Similar to this finding, many plant extracts possess beneficial effect in the prevention of hyperglycemia induced muscle wastage [52-54] and similar result was observed in this study.
Antioxidant capacity hydromethanolic seed extract of Stramonium was determined in 2,2-diphenyl-1-picrylhydrazine (DPPH) [55]. Free radical scavenging property of seed extract was concentration dependent and comparable with standard antioxidant (ascorbic acid). Similar finding reported that strong antioxidant activity of leaf latex and isolated compound of Aloe schelpei,[56] and leaf latex of Aloe megalacantha [49] in 2, 2-diphenyl-1-picrylhydrazyl (DPPH) assay. Plant derived supplement prevent devastating effect of oxidative stress (ROS) in chronic disease like DM and Datura stramonium might offer health benefit through antioxidant property in chronic diseases.
Phytomolecules have been demonstrated blood glucose lowering activity through different mechanisms. They increase glucose up take by augmenting insulin action [57] and increase GLUT 2 expression and promoting translocation GLUS-4 by flavonoids, [58] increase insulin secretion, [59] reduced carbohydrate digestion and absorption by inhibiting α-glucosidase and α-amylase, [45,58,60,61] augment β-cells proliferation and regeneration [45,59] preventing pancreatic β-cells dysfunction through free radical scavenging action by tannins and phenols like flavonoids [62-64] or by any other unknown mechanisms. In the present finding, the preliminary phytochemical analysis of the hydromethanolic seed extract of D. stramonium has phenolic compounds, anthraquinones, glycosides, saponins, terpenoids, tannins and flavonoids. Therefore, the antidiabetic of the hydromethanolic seed extract could elicit a single or synergetic action of these metabolites.
Conclusion
The finding of the study showed that hydromethanolic seed extract of Datura Stramonium endowed significant blood glucose lowering and antioxidant activity. Further studies are required for bioassay guided fractionation, isolation and characterization of active compound (s) that possess glucose lowering activity.
Declarations
Ethics approval
The study was conducted according to OECD Guidelines and the Guide for the Care and Use of Laboratory Animals. Ethical approved was obtained from the ethical review committee of School of pharmacy, college of medicine and health sciences, Wollo University.
Availability of Data and Materials
All the datasets used/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declared that they do not have any conflict of interest.
Funding
Not applicable
Authors Contributions
BC and GG have contributed to conception, design, analysis and interpretation of data, drafting the article and revising the manuscript and gave final approval for publication.
Acknowledgments
We would like to acknowledge Wollo University for providing the necessary facilities in this study.
REFERENCES
- Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66:241-255.
- Alberti KG, Zimmet PF. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic med. 1998;15:539-553.
- Schofield JD, Liu Y, Rao-Balakrishna P, Malik RA, Soran H. Diabetes dyslipidemia. Diabetes Therapy. 2016;7:203-219.
- Vijayaraghavan K. Treatment of dyslipidemia in patients with type 2 diabetes. Lipids in Health and disease. 2010;9:1-2.
- Edition ID. International Diabetes Federation. IDF Diabetes Atlas, 8th edn. Brussels, Belgium: International Diabetes Federation, 2017.
- Varas-Lorenzo C, Margulis AV, Pladevall M, Riera-Guardia N, Calingaert B, Hazell L, et al. The risk of heart failure associated with the use of noninsulin blood glucose-lowering drugs: systematic review and meta-analysis of published observational studies. BMC cardiovascular disorders. 2014;14:129.
- Rao MU, Sreenivasulu M, Chengaiah B, Reddy KJ, Chetty CM. Herbal medicines for diabetes mellitus: a review. Int J PharmTech Res. 2010;2:1883-1892.
- Tschape D, Hanefeld M, Meier JJ, Gitt AK, Halle M, Bramlage P, et al. The role of co-morbidity in the selection of antidiabetic pharmacotherapy in type-2 diabetes. Cardiovascular diabetology. 2013;2:1.
- Abdissa D, Geleta G, Bacha K, Abdissa N. Phytochemical investigation of Aloe pulcherrima roots and evaluation for its antibacterial and antiplasmodial activities. PloS one. 2017;12.
- Yousef F, Yousef N, Mansour O, Herbali J. Metformin: a unique herbal origin medication. Global J Med Res. 2017;17.
- Hung H-Y, Qian K, Morris-Natschke SL, Hsu C-S, Lee K-H. Recent discovery of plant-derived anti-diabetic natural products. Natural product reports. 2012;29:580-606.
- Goel PK. Novel antidiabetic furostanolic saponin rich (fsr) fraction from fenugreek seeds. United States patent application US 13/322,460. 2012.
- Samuels J. Biodiversity of food species of the Solanaceae family: a preliminary taxonomic inventory of subfamily Solanoideae. Resources. 2015;4:277-322.
- Gebhardt C. The historical role of species from the Solanaceae plant family in genetic research. Theoretical and Applied Genetics. 2016;129:2281-2294.
- Al-Snafi AE. Medical importance of Datura fastuosa (syn: Datura metel) and Datura stramonium-A review. IOSR Journal of Pharmacy. 2017;7:43-58.
- Soni P, Siddiqui AA, Dwivedi J, Soni V. Pharmacological properties of Datura stramonium L. as a potential medicinal tree: An overview. Asian Pacific J of tropical biomed. 2012;2:1002-1008.
- Miraj S. Datura stramonium: An updated review. Der Pharma Chem. 2016;8:253-257.
- Lewis O, Nieman E, Munz A. Origin of amino acids in Datura stramonium seeds. Annals of Botany. 1970;34:843-848.
- Meresa A, Gemechu W, Basha H, Fekadu N, Teka F, Ashebir R, et al. Herbal Medicines for the Management of Diabetic Mellitus in Ethiopia and Eretria including their Phytochemical Constituents. American J of Adv Drug Delivery. 2017;5:1040-1058.
- Rashid S, Ahmad M, Zafar M, Anwar A, Sultana S, Tabassum S, et al. Ethnopharmacological evaluation and antioxidant activity of some important herbs used in traditional medicines. J Traditional Chinese Med. 2016;36:689-94.
- Baynesagne S, Berhane N, Sendeku W, Ai L. Antibacterial activity of Datura stramonium against standard and clinical isolate pathogenic microorganisms. J Medi Plants Res. 2017;11:501-506.
- Abbas DA. Analgesiac, anti-inflammatory and antidiarrheals effects of Datura stramonium hydroalcoholic leaves extract in mice. IJRRAS. 2013;14:193-199.
- Shobha G, Soumya C, Shashidhara KS, Moses V. Phytochemical profile, antibacterial and antidiabetic effects of crude aqueous leaf extract of Datura stramonium. Pharmacophore. 2014;5:273-278.
- Mehrabadi M, Bandani A, Saadati F, Mahmudvand M. α-Amylase activity of stored products insects and its inhibition by medicinal plant extracts. Journal of Agricultural Science and Technology. 2011;13:1173-1182.
- Belayneh YM, Birhanu Z, Birru EM, Getenet G. Evaluation of in vivo antidiabetic, antidyslipidemic, and in vitro antioxidant activities of hydromethanolic root extract of Datura stramonium L. (Solanaceae). J Exp Pharmacol. 2019;11:29.
- National Research Council. Guide for the care and use of laboratory animals. National Academies Press. 2010.
- Pandey A, Tripathi S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J Pharmacogn Phytochem. 2014;2.
- Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Int Pharmaceutical Sci. 2011;1:98-106.
- Organization for Economic Co-Operation and Development. OECD Guideline for the testing of chemicals: acute oral toxicity-Up-and-down procedure. 2000.
- MacDonald‐Wicks, Wood LG, Garg ML. Methodology for the determination of biological antioxidant capacity in vitro: a review. Journal of the Science of Food and Agriculture. 2006; 86:2046-2056.
- Etuk E. Animal models for studying diabetes mellitus. Agric Biol J N Am. 2010;1:130-134.
- MMPC. Streptozotocin-induced type 1 diabetes model. Mouse Metabolic Phenotyping Centers protocols. 2013.
- Sulyman AO, Akolade J, Sabiu S, Aladodo R, Muritala H. Antidiabetic potentials of ethanolic extract of Aristolochia ringens (Vahl.) roots. Journal of Ethnopharmacol. 2016;182:122-128.
- Baquer NZ, Kumar P, Taha A, Kale RK, Cowsik SM, McLean P, et al. Metabolic and molecular action of Trigonella foenum-graecum (fenugreek) and trace metals in experimental diabetic tissues. J Biosci. 2011;36:383-396.
- Tamiru W, Engidawork E, Asres K. Evaluation of the effects of 80% methanolic leaf extract of Caylusea abyssinica (fresen.) fisch. & Mey. on glucose handling in normal, glucose loaded and diabetic rodents. BMC complementary and alternative medicine. 2012;12:151.
- Demoz MS, Gachoki KP, Mungai KJ, Negusse BG. Evaluation of the anti-diabetic potential of the methanol extracts of aloe camperi, meriandra dianthera and a polyherb. J of Diab Melli. 2015;5:267.
- Bowe JE, Franklin ZJ, Hauge-Evans AC, King AJ, Persaud SJ, Jones PM, et al. Metabolic phenotyping guidelines: assessing glucose homeostasis in rodent models. J of Endocrinol. 2014;222:G13-G25.
- Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Disease models & mechanisms. 2010;3:525-534.
- Zhang P. Glucose tolerance test in mice. Bio-protocol Bio. 2011;101:e159.
- Sharma S, Choudhary M, Bhardwaj S, Choudhary N, Rana AC. Hypoglycemic potential of alcoholic root extract of Cassia occidentalis Linn. in streptozotocin induced diabetes in albino mice. Bulletin of Faculty of Pharmacy, Cairo University. 2014;52:211-217.
- Sharma B, Salunke R, Balomajumder C, Daniel S, Roy P. Anti-diabetic potential of alkaloid rich fraction from Capparis decidua on diabetic mice. Journal of Ethnopharmacol. 2010;127:457-462.
- Zhang Y, Feng F, Chen T, Li Z, Shen QW. Antidiabetic and antihyperlipidemic activities of Forsythia suspensa (Thunb.) Vahl (fruit) in streptozotocin-induced diabetes mice. Journal of Ethnopharmacol. 2016;192:256-263.
- Rajurkar B. Phyto-pharmacological investigations of Clerodendrum infortunatum Gartn. International Res J of Pharm. 2011;2:130-132.
- Tesfaye A, Makonnen E, Gedamu S. Hypoglycemic and antihyperglycemic activity of aqueous extract of Justicia Schimperiana leaves in normal and streptozotocin-induced diabetic mice. Inte J of Phar Scis and Res. 2016;7:110-113.
- Toma A, Makonnen E, Mekonnen Y, Debella A, Adisakwattana S. Antidiabetic activities of aqueous ethanol and n-butanol fraction of Moringa stenopetala leaves in streptozotocin-induced diabetic rats. BMC complementary and alternative medicine. 2015;15:242.
- Otsuka H. Purification by solvent extraction using partition coefficient. In Natural Products Isolation 2006;269-273.
- Belayneh YM, Birru EM, Ambikar D. Evaluation of hypoglycemic, antihyperglycemic and antihyperlipidemic activities of 80% methanolic seed extract of Calpurnia aurea (Ait.) Benth (Fabaceae) in mice. J of Exp Pharmacol. 2019;11:73.
- Lanjhiyana S, Garabadu D, Ahirwar D, Rana AC, Ahirwar B, Lanjhiyana SK, et al. Pharmacognostic standardization and hypoglycemic evaluations of novel polyherbal formulations. Der Pharm Let. 2011;3:319-333.
- Hammeso WW, Emiru YK, Ayalew Getahun K, Kahaliw W. Antidiabetic and antihyperlipidemic activities of the leaf latex extract of Aloe megalacantha baker (Aloaceae) in streptozotocin-induced diabetic model. Evidence-Based Complementary and Alternative Medicine. 2019.
- Manjunath K, Subash KR, Tadvi NA, Manikanta M, Rao U. Effect of Aloe vera leaf extract on blood glucose levels in alloxan induced diabetic rats. Natl J Physiol Pharm Pharmacol. 2016;6:471-474.
- Murthy BK, Nammi S, Kota MK, Rao RK, Rao NK, Annapurna A. Evaluation of hypoglycemic and antihyperglycemic effects of Datura metel (Linn.) seeds in normal and alloxan-induced diabetic rats. J of Ethnopharmacol. 2004;91:95-98.
- Eleazu CO, Iroaganachi M, Okafor PN, Ijeh II, Eleazu KC. Ameliorative potentials of ginger (Z. officinale Roscoe) on relative organ weights in streptozotocin induced diabetic rats. International journal of biomedical science: IJBS. 2013; 9:82.
- Stirban A, Gawlowski T, Roden M. Vascular effects of advanced glycation endproducts: clinical effects and molecular mechanisms. Molecular Metabol. 2014;3:94-108.
- Choudhury H, Pandey M, Hua CK, Mun CS, Jing JK, Kong L, et al. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J Tradit Complement Med. 2018;8:361-376.
- Moon JK, Shibamoto T. Antioxidant assays for plant and food components. J Agricul and Food chem. 2009; 57:1655-1666.
- Teka T, Kassahun H. Characterization and Evaluation of Antioxidant Activity of Aloe schelpei Reynolds. Drug Design, Development and Therapy. 2020;14:1003-1008.
- Choudhary M, Kochhar A, Sangha J. Hypoglycemic and hypolipidemic effect of Aloe vera L. in non-insulin dependent diabetics. Journal of food science and technology. 2014;51:90-96.
- Romano B, Pagano E, Montanaro V, Fortunato AL, Milic N, Borrelli F, et al. Novel insights into the pharmacology of flavonoids. Phytotherapy Res. 2013;27:1588-1596.
- Sayem AS, Arya A, Karimian H, Krishnasamy N, Ashok Hasamnis A, Hossain CF, et al. Action of phytochemicals on insulin signaling pathways accelerating glucose transporter (GLUT4) protein translocation. Molecules. 2018;23:258.
- Tekulu GH, Araya EM, Mengesha HG. In vitro α-amylase inhibitory effect of TLC isolates of Aloe megalacantha baker and Aloe monticola Reynolds. BMC complementary and alternative medicine. 2019;19:206.
- Pereira DF, Cazarolli LH, Lavado C, Mengatto V, Figueiredo MS, Guedes A, et al. Effects of flavonoids on α-glucosidase activity: potential targets for glucose homeostasis. Nutrition. 2011;27:1161-1167.
- Ayoola GA, Coker HA, Adesegun SA, Adepoju-Bello AA, Obaweya K, et al. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop J of Pharmaceutical Res. 2008;7:1019-1024.
- Ifesan BO, Fashakin JF, Ebosele F, Oyerinde AS. Antioxidant and antimicrobial properties of selected plant leaves. Europ J of Medi Plant. 2013:465-473.
- Oboh G, Rocha JB. Polyphenols in red pepper [Capsicum annuum var. aviculare (Tepin)] and their protective effect on some pro-oxidants induced lipid peroxidation in brain and liver. Europ Food Res and Technol. 2007;225:239-247.
Citation: Melaku BC, Getnet G (2020) Evaluation of Antidiabetic and Antioxidant Potential of Hydromethanolic Seed Extract of Datura Stramonium in Mice. J Clin Exp Pharmacol. 10:267. doi: 10.35248/2161-1459.20.10.267
Copyright: © 2020 Melaku BC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited


