PMC/PubMed Indexed Articles
Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 0, Issue 0
Evaluation of a Combined Peste des Petits Ruminants and Rift Valley Fever Live Vaccine in Sheep and Goats
N Safini1, Z Bamouh2, J Hamdi2, K O Tadlaoui2, D M. Watts1* and M El. Harrak22Research and Development Virology, Multi-Chemical Industry (MCI) Santé Animale, Mohammedia, Morocco
Received: 08-Jun-2021 Published: 29-Jun-2021, DOI: 10.35248/2157-7560.21.s14.003
Abstract
Small Ruminants (SR) are an important source of nutrition and economies for sustaining the livelihoods of vulnerable human population in many rural regions of Africa. The health of these animals continues to be threatened by the highly infectious trans boundary viral diseases, such as Peste des Petit Ruminants (PPR) and Rift Valley fever (RVF) as well as other infectious diseases that cause high rates of disease and death in these animals. Therefore, the objective of this study was to develop and evaluate a combined vaccine for PPR and RVF in sheep and goats in Morocco. The vaccine was prepared by propagating the viruses together in Vero cells and the selection of different infectious doses for conducting vaccine trials in the animals. Safety was assessed based on rectal temperature, clinical manifestations and possible adverse reaction at the vaccine injection site. Immunogenicity was determined by testing sera samples obtained Post-Vaccination (PV) for PPRV and RVFV ELISA IgG and serum neutralizing antibodies. The results indicated that the vaccine was safe and immunogenic in the animals with minimal auto-interference between the 2 viruses. Sheep vaccinated with different doses of the combined PPRV/RVFV vaccine developed detectable PPRV antibody by day 7 pv, including 80% of the groups of animals that received the same doses and 100% that received a low and high dose. In the goats that received the combined PPRV/RVFV, RVFV antibody was detected in all vaccinated animals at day 7 pv, including 70% of the animals that received the low dose, 100% that received the high dose and 20% of the animals that received the same dose. Our finding revealed that the combined live PPRV/RVFV vaccine can be used safely as a one dose vaccination in sheep and goat for large vaccination campaign to prevent both PPRV and RVFV diseases in enzootic countries.
Keywords
PPRV; RVFV; Small ruminants; Vaccine
Introduction
Small Ruminants (SR) play an important role as a source of nutrition and economies in sustaining the livelihoods of vulnerable population in many rural regions of Africa [1-2]. The health of these animals continues to be threatened by the highly infectious transboundary viral disease Peste des Petit Ruminants (PPR) and to a lesser extent by Rift Valley Fever (RVF) as well as other viral pathogens [3-7]. PPR, also known as goat plague or Morbillivirus infection of SR is a viral disease of goats and sheep characterized by fever, sores in the mouth, diarrhea, pneumonia with morbidity and mortality rates that can be as high as 90% [8]. The disease is caused by Peste-des-Petits-Ruminants virus (PPRV) of the family Paramyxoviridae, subfamily Orthoparamyxovirinae, genus Morbillivirus, species Small ruminant morbillivirus [8] which is related to but distinct from Rinderpest virus [9]. Since PPR was first reported in 1942 in the Ivory Coast, the disease has spread far beyond its origin in Western Africa. In the past 15 years, the PPRV has been reported in over 70 countries across Africa, the Near and Middle East and Asia, reaching Eastern Europe in 2016. RVF is a viral zoonosis disease that affects both animals and humans by causing mild to severe disease. The causative agent, Rift Valley Fever Virus (RVFV) is a member of the order Bunyavirales, family Phenuiviridae, genus Phlebovirus, species Rift Valley fever phlebovirus [10]. The disease causes significant health and economic losses among domestic ruminants due to abortions and teratogenic effect in pregnant females and high mortality in young animals. Since the discovery of RVFV during 1931 in eastern African the virus has emerged throughout most of continental Africa as well as Madagascar and the Arabian Peninsula [11].
Several vaccines have been shown to prevent RVF and PPR among domestic ruminants in Africa [12]. The attenuated PPR Nigeria 75 virus strain was used to produce a vaccine that conferred good protection against the PPR in SR [13]. Despite PPR vaccination efforts in many countries, the coverage rate has been very low this could limit the goal toward of eradicating PPR disease the year 2030 [14]. The RVFV Clone 13 (CL13) is a natural live attenuated RVFV mutant, which was isolated from a non-fatal human case of RVF [15]. CL13 has a large deletion in the Non-structural protein coded by the S segment (NSs), which attenuated this protein as a virulence factor [16]. The attenuated RVFV Clone 13 vaccine has been shown to protect sheep, goats, cattle and camels against the RVF [17]. However, vaccination coverage is not sustained for RVF in enzootic areas and vaccines are only administered when this disease emerges to cause epizootics and epidemics. A combined RVFV and PPRV vaccine will offer an approach to sustain vaccination coverage for both diseases with the goal of eradicating PPR by 2030 [18]. Therefore, the objective of this study was to develop and evaluate a combined PPRV and RVFV vaccine for small ruminants to prevent disease in domestic animals and minimize vaccination costs and to improve vaccination coverage and efficacy.
Materials and Methods
In-vitro PPRV/RVFV co-culture
Viral strains: The attenuated RVFV Clone 13T virus [19,20] and the PPRV vaccine virus strain, Nigeria 75 (PPR N75) previously reported were used in this study.
Cell lines: Vero cells (African green monkey kidney), American Type Tissue Culture, Manassas, VA 20110, U.S.A. were used for growth and titration of PPRV and RVFV. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) Wisent Inc., ST- BRUNO, Quebec, Canada J3V 4P8 with 10% Fetal Bovine Serum (FBS) Wisent Inc., ST-BRUNO, Quebec Canada J3V 4P8 for growth and 1% FBS when inoculating sheep and goats with RVFV and PPRV.
Infection of cells: The infectivity titer of PPRV and RVFV vaccines was determined by co-replication in Vero cells following Multiplicities of Infection (MOI) PPRV/RVFV: 0.01/0.01 and 0.01/0.001. The infected cells were examined microscopically daily. When the Cytopathic effect was 80%, the virus suspension was harvested and frozen at -80°C. The suspension was then thawed at room temperature and the virus suspension was used for passage P2 that was performed with the P1 harvest without an estimate of the MOI, and the same was performed for passage 3 (P3). The infectivity titers and quantitative Polymerase Chain Reaction (qPCR) counts (Ct) were determined for each passage of the RVFV and the PPRV by titration using the Immuno-Fluorescence Assay (IFA).
Immuno-Fluorescence Assay: The plates of Vero infected cells that were inoculated with ten-fold dilutions of the combined PPRV/ RVFV were formalin fixed and permeabilized with 0.1% Triton X-100 in Phosphate-Buffered Saline (PBS) for 20 min. Monoclonal antibodies, including RVFV Gn-Gc (Alpha Diagnostic Intl. Inc.| San Antonio, Texas 78249 USA) and PPRV mouse monoclonal antibody IgG (Mab 2.15 gift from AU-PANVAC, Debre Zeit, Ethiopia) were used as primary antibodies. The binding of primary antibody to the virus infected cells was detected by an anti-species IgG-Fluorescein Isothiocyanate (FITC) or Texas Red dye conjugate (Bethyl Laboratories, Inc., Montgomery, TX 7735) under an inverted fluorescence microscope (Optika Srl Via Rigla,30–24010 Ponteranica (Italy)). The highest dilution with viral specific fluorescence was considered as the virus titer and was calculated by the Reed and Muench Tissue Culture Infectious Dose 50% (TCID50) method [21].
Giemsa staining: Vero cells were inoculated with PPRV and RVFV separately and together with both PPRV/RVFV. After 3 days of incubation, cells were fixed for 5 min with ice-cold methanol and air dried. After removing methanol, cells were covered with Giemsa stain (4% v/v Giemsa-stain in double-distilled water) for 10 minutes, rinsed with several changes of tap water, air dried, and examined by light microscopy at 400 X to visualize Cytopathic Effect (CPE) such as vacuolation and viral inclusion bodies.
Quantitative real-time PCR: Viral charge (RNA) of the PPRV and RVFV was determined by a real time Polymerase Chain Reaction (qPCR) assay using aliquots of the ten-fold dilutions of infected Vero cells as an estimate of the titer. Total viral RNA was extracted from infected Vero cells by using Isolate II RNA mini kit (Bioline Life Science Division of Meridian Bioscience Memphis, Tennessee 38134-5611 USA). The extracted RNA was eluted in a volume of 60 μl of buffer and stored at -20°C until use. PPRV viral charge detection was performed using primer and probe. Specific for Nucleocapsid (N) gene [22]. Briefly, each 25 μl reaction contained 4 μl of extracted RNA; 12.5 μl of 2x Sensi FAST Probe Lo-ROX One-Step Mix; 0.5 μl probe (10 μM); 1 μl (10 μM) forward and reverse primer and 6 μl nuclease free water. The cycling conditions were as follows: reverse transcription 45°C for 10 min; reverse transcription inactivation and DNA polymerase activation 95°C for 2 min; followed by 40 cycles of amplification (95°C for 5 s and 60°C for 20 s). The results were generated by determination of the threshold cycle (Ct).
A one-step real time PCR assay was used to determine the copies of RVFV genome in a volume of 20 μl as an estimate of the titer. The primer-probe was set to target the L-segment [23]. Tests were performed in 96-well Optical Reaction Plates (Applied Biosystems, Foster City, CA 9440 U.S.A.) that contained 10 μl Sensi FAST Probe Lo-ROX one step Master Mix, 500 nM of each primer, and 250 nM of fluorogenic probe, 4 μl of template. Reactions were run using the following amplification program: reverse transcription at 45°C for 15 min, and initial denaturation at 95°C for 2 min, 45 cycles with 95°C for 5 s and 60°C for 30 s. Fluorescence was read at the combined annealing-extension step at 60°C.
Vaccine preparation and stability study: The RVFV and PPRV vaccine virus strains were grown separately in confluent monolayers of Vero cells in roller bottles using the same M.O.I. of 0.01. Viral suspensions were harvested when CPE was observed to be 80% on day 4 post-inoculation of cells for both PPRV and RVFV. The viral suspension was then stored at -80°C until used to prepare vaccines. These two preparations of viruses were used to prepare a monovalent PPRV vaccine, a monovalent RVFV vaccine and combined PPRV/RVFV vaccines as lyophilized forms. The first passage of the PPR -N75 and RVFV clone 13T viruses were used as live vaccine antigen cultured in Vero cells and were both mixed and added to an equal volume of stabilizer (peptone, sucrose and glutamate) and then lyophilized. For vaccination, the freeze- dried vaccine vial of 100 doses was reconstituted in 50 ml of the diluent (saline solution) for a vaccine dose of 0.5. The vaccine was formulated in appropriate concentration of antigens according to their respective titers 106.7 TCID50/ml for PPR N75 and 108.6 TCID50/ml for RVFV. The infectivity of the combined vaccines was tested to determine stability during storage for 18 months at 4°C. Each month, two vials of the freeze-dried vaccine were pooled, and then 10-fold dilutions were prepared and titrated in Vero cells as described above to determine the infectivity titers using the Reed-Muench method and expressed as TCID50/ml [21].
Vaccination of animals: All animals used in this study included a local breed of sheep and Alpine goats. Trials were carried out in accordance with an animal care and use protocol approved by the MCI ethics committee and are following ARRIVE (spell-out and then abbreviate) guidelines. Vaccination of all animals was conducted in a Biosafety level 3 large animal holding facilities. Animals were all confirmed to be free of antibodies to RVFV and PPRV by ELISA and a Virus-Neutralization Test (VNT) as described below in the OIE Manual (OIE Chapters 2.7.11 and 2.7.14). Commercialized test kits were used to test animal sera samples for PPRV antibodies and RVFV IgG antibodies, respectively.
A total of 47 sheep and 15 goats that ranged from 3-6 months of age were used in the vaccine trials (Table 1). Of these animals, a total of 30 sheep was randomly divided into 3 groups (1 to 3) of 10 sheep each and vaccinated subcutaneously (SC) with different doses of the combined PPRV/RVFV vaccine. In group 4, 10 goats were inoculated with different doses of the combined PPRV and RVFV vaccines. Two groups of 4 sheep each (groups 5 and 7) were vaccinated with the PPRV and RVFV monovalent vaccines with the minimal dose used in group 1 and monovalent control for group 2. Another two groups (6 and 8) of 4 goats each were vaccinated with monovalent PPRV and RVFV vaccine with similar doses used in group 4 (PPRV: 103.3 and RVFV: 103.2). The controls (8 sheep and 5 goats) were vaccinated with saline and housed in the same pen as the vaccinated animals. Vaccinated and unvaccinated animals were observed, and blood samples were obtained once a month for a period of 3 months to one year. Animals used in Groups 1 and 4 were maintained in the animal holding facility for a period of 12 months, respectively and the other animals were observed for another month and then euthanized. A dose of 104.5 was used as an overdose for the combined vaccines in sheep for RVFV and 104 for PPRV. Doses of 103.2 and 103.3 were used as overdoses in the goats for RVFV and PPRV respectively. After vaccination, blood samples were obtained in a volume of 5 ml on day 1, 2 and 3 from the jugular vein of each animal to test for virus using a 20 gauge needle and 10 ml syringe. Also, on the same days, nasal and rectal samples were collected for nucleic acid PCR test in groups vaccinated with PPRV. Rectal temperatures and general behavior of the animals was recorded daily for 14 days. Animals were also monitored daily for clinical signs including weakness, respiratory signs and local inflammation at the injection site. Blood samples were obtained to provide serum samples to test for PPRV and RVFV antibodies weekly as described above for 8 weeks and then monthly, ranging from 3 months to one year.
| Vaccine | Group | Number of animals | Vaccine dose |
|---|---|---|---|
| Combined PPRV/RVFV | 1 | 10 Sheep | PPR: 102.5 |
| RVF: 103.5 | |||
| 2 | 10 Sheep | PPR: 103.5 | |
| RVF: 104.5 | |||
| 3 | 10 Sheep | PPR: 104.0 | |
| RVF: 104.1 | |||
| 4 | 10 Goats | PPR: 103.3 | |
| RVF: 103.2 | |||
| Monovalent PPRV | 5 | 4 Sheep | PPR: 102.5 |
| 6 | 4 Goats | PPR: 103.3 | |
| Monovalent RVFV | 7 | 4 Sheep | RVF: 103.5 |
| 8 | 4 Goats | RVF: 103.2 | |
| Unvaccinated | 9 | 8 Sheep | Saline Placebo- |
| animals | 10 | 5 Goats | Saline Placebo |
Abbreviations: RVFV: Rift Valley Fever Vaccine; PPRV: Peste des Petit Ruminants Vaccine
Table 1: Vaccination of sheep and goats with different doses of the combined PPRV/RVFV and a single dose of the monovalent PPRV and RVFV vaccines with control groups.
Serological tests: Sera were screened for RVFV and PPRV: PPR b-ELISA from AU-PANVAC and for RVFV antibodies with commercial competitive ELISA kit (RVF-ID Screen) from IDvet, Grabels, France, and PPRV using PPR b-ELISA kit purchased from AU-PANVAC, Debre Zeit, Ethiopia. Testing was performed according to the manufacturer’s instructions and results were read at a wavelength of 450 nm.
The VNT was based on the performance of serial ¼ dilutions of heat-inactivated sera and a constant amount of infectious virus (100 TCID50). The neutralizing antibody titer was calculated as the highest dilution to produce at least 90% neutralization in accordance with the Reed and Muench method [21]. Sera were tested simultaneously with both the ELISA and VNT.
Statistical analysis: All results were calculated and presented as the means ± standard error of the mean obtained from triplicate tests. The statistical significance was determined by one-way or two-way analysis of variance. P values of <0.05 were considered as statistical significance.
Results
Assessment of possible replication interference between PPRV/RVFV in Vero cells
The susceptibility of Vero cells to PPRV and RVFV infection was evaluated by the appearance of Cytopathic Effect (CPE) and the amount of replicated virus. When the cells were inoculated separately, PPRV induces typical syncytial effect, whereas RVFV caused CPE that was observed as visible dead floating cells much faster than PPRV induced CPE, one-day vs. 2-3 days’ pi (Figure 1). However, the cells inoculated with passage 1 of the combined PPRV/RVFV exhibited CPE as floating dead cells typical RVFV CPE, while for passage 2 of the combined viruses, cells appeared in clusters (Figure 1).
Figure 1: Cytopathic effect of individual PPR and RVF viruses and cytopathic effect for co-infection with both viruses in Vero cell culture. PPRV CPE is characterized by syncytia (a multinucleate cell which can result from multiple cell fusions of uninuclear cells), RVFV CPE by rounding cells and fast destruction of the monolayer. original magnification at X 400.
PPRV produces characteristic cytopathogenicity as large number of syncytia assembled many cells inside the cytoplasm. The syncytia were followed by necrotic foci (dark blue cytoplasm). When the combined PPRV and RVFV were inoculated onto Vero cells, RVFV induced CPE was dominant and no syncytia (PPRV CPE) were observed for the 3 different passages even after 6 dpi (Figure 2). Immunofluorescence showed that both PPRV and RVFV were localized in the cytoplasm (data not shown).
Figure 2: Giemsa staining of Vero cells inoculated separately with PPRV, RVFV and both PPRV/RVFV at MOI=0.01 after 72h incubation. Pictures were taken under optical microscope with an original magnification at: x100, x400 and x1000. Arrows are pointing to PPR CPE Syncytia (multinucleated cell). Arrowheads indicate RVFV CPE: necrotic foci (necrotic rounding cells with cytoplasm in dark blue, cells are shrinked/dead).
Infectivity of the PPRV/RVFV in cells
The incubation period for the peak replication of the combined PPRV and RVFV in Vero cells occurred within 4 days. The peak level of PPRV replication was 106.2 TCID 50/ml in 4 days. Whereas the peak titers for RVFV of 108.6 TCID 50/ml occurred on day 3 and further cultivation reduced the infectivity titer to 108 TCID 50/ml on the 4th day. Hence the optimal harvest day for both viruses was on day 4 pi (Figure 3).
Figure 3: Infectious titers at daily intervals of combined PPRV/RVFV in Vero cells infected with same MOI 0.01/0.01. Data shown are means of at least three independent experiments (n=3). D: Day.
As presented in (Figure 4A), using an inoculum of a MOI of 0.01 for PPRV, the infectivity titers decreased by 1 log10 with each passage (p<0.05) Whereas the titer of the RVFV remained at 7.6 TCID50/ml over 3 passages (p<0.05) The use of a lower MOI for RVFV (0.001) did not solve the decrease in the infectivity titer of the PPRV and the RVFV titers remained high during the 3 passages (Figure 4B). Interestingly, when we inoculated cells with PPRV with MOI 0.1 and RVFV with lower MOI 0.01, PPR N75 virus could replicate consistently with higher titer compared with other lower tested MOI when coinfected with RVFV (Figure 4C).
Figure 4(A-D): Infectious titers (TCID 50/ml) and viral charge of PPRV and RVFV after co-infection in 3 passages (P) in Vero cells. A-Infectious titers of PPRV and RVFV coinfected at same MOI 0.01 in 3 successive passages B-Infectious titres of PPRV ans RVFV coinfected at different MOI 0.01 of PPRV and 0.001 of RVFV in 3 successive passages. C- Infectious titres of PPRV and RVFV coinfected at MOI 0.1 of PPRV and 0.01 of RVFV in 3 successive passages. D- Viral PCR charge (Ct) of PPRV and RVFV in 3 passages. Data shown are means of at least three independent experiments (n=3).
These results were confirmed by qPCR which showed Ct to be a 4 to 5 folds increase between 1st and 2nd passage especially with PPRV, while at MOI 0.1 PPRV Ct was 10 times lower than in the other tested MOI. Concerning the Ct of RVFV, viral charges remain similar during the 3 successive passages in all tested MOI (Figure 4D).
Safety testing of combined PPRV/RVFV vaccine
The safety of the combined and monovalent vaccines was evaluated in sheep and goats by monitoring the post-vaccination clinical manifestations. The vaccine was safe as indicated by normal body temperatures in all the goats and sheep vaccinated with different doses of the vaccines (Figures 5 and 6). Also, during the 15 days’ post-vaccination period, none of the animals exhibited abnormal behavior, nor were there any clinical signs or local reactions at the injection sites (data not shown). No nucleic acids upon qPCR testing neither for PPRV nor for RVFV was detected in blood or swabs.
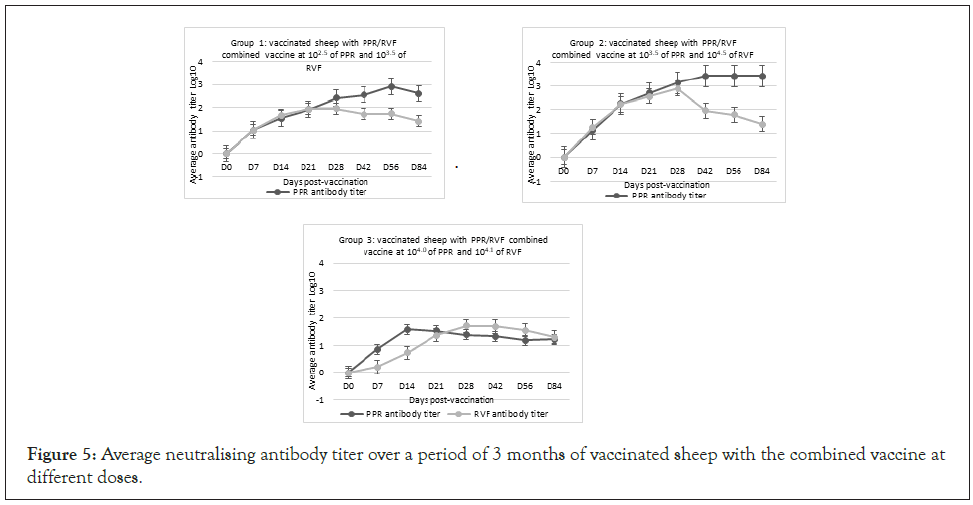
Figure 5: Average neutralising antibody titer over a period of 3 months of vaccinated sheep with the combined vaccine at different doses.
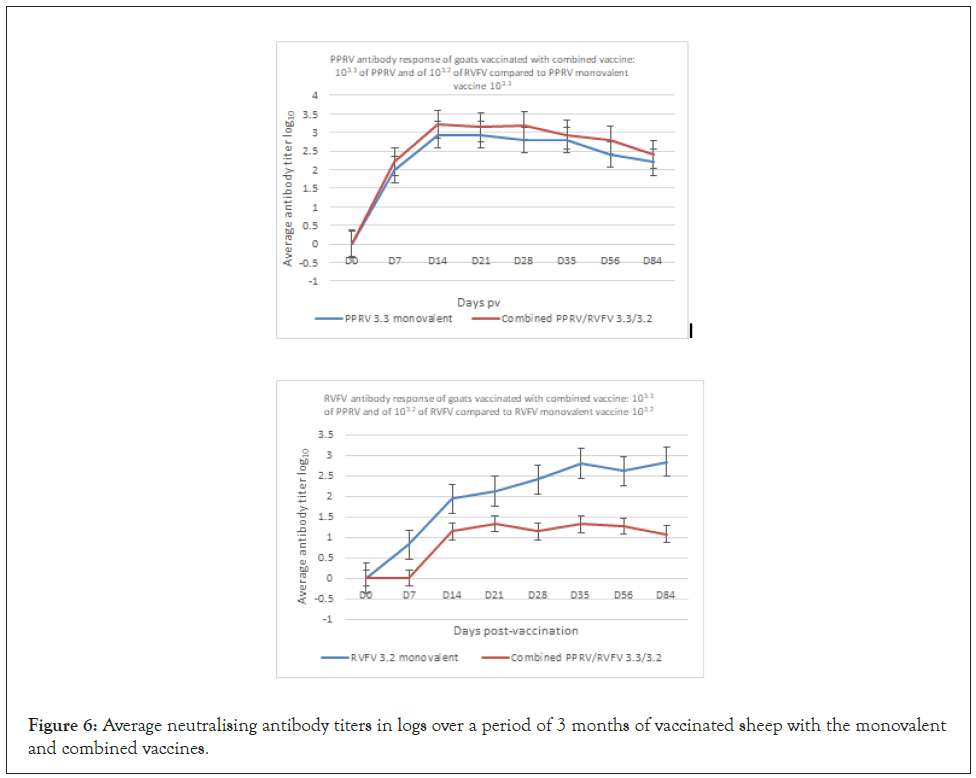
Figure 6: Average neutralising antibody titers in logs over a period of 3 months of vaccinated sheep with the monovalent and combined vaccines.
Serological responses of sheep vaccinated with PPRV/ RVFV
The immunogenicity of combined PPRV/RVFV vaccine was evaluated by vaccinating 3 groups of 10 sheep each SC with 3 different doses of the vaccine, including low dose (102.5 of PPRV and 103.5 of RVFV), high dose (103.5 of PPRV and 104.5 of RVFV) and same doses (10.4.0 of PPRV and RVFV) for group 1, 2 and 3, respectively (Table 1). Also, the immunogenicity of both RVFV and PPRV was determined for sheep vaccinated with monovalent PPRV/RVFV vaccines.
Sheep vaccinated at different doses of the combined PPRV/ RVFV vaccine developed detectable PPRV antibody by day 7 pv, including 80% of the animals that received the same doses and 100% that received low and high doses (Table 2). High titers of PPRV antibodies were recorded in vaccinated sheep from group 1 and 2 at day 56 and 84 pv with a value of 3.42 and 2.9 log10, respectively. In group 3, the peak neutralizing antibody titer was observed on day 14 pv (1.58 log10) and all animals had developed PPRV neutralizing antibody (Figure 5). For the group of sheep that received the combined PPRV/RVFV, antibody against RVFV was detected in all vaccinated animals at day 7 pv, including 70% of the animals that received the low dose, 100% of animals that received the high dose and 20% of the animals that received the same dose (Table 2). The maximum antibody titer was observed on day 28 pv with peak neutralizing titers of 1.95, 2.9 and 1.9 log10 in sheep, groups 1, 2 and 3, respectively (Figure 5). Sheep vaccinated with monovalent PPR and RVF vaccines seroconverted based on the detection of both VNT and ELISA antibodies by day 7 pv. The high titers of neutralizing antibodies in all groups were maintained for a period of 3 months (Figure 6). All unvaccinated controls remained negative indicating an absence of spread of the vaccine virus between the groups of vaccinated and unvaccinated animals (Table 2).
| Days post-vaccination | D0 (Day 0 pv) | D7 | D14 | D21 | D28 | D42 | D56 | 3M | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccine/Group of animals | Anti-PPRV | Anti- | Anti-PPRV | Anti-RVFV | Anti-PPRV | Anti-RVFV | Anti-PPRV | Anti-RVFV | Anti-PPRV | Anti-RVFV | Anti-PPRV | Anti-RVFV | Anti-PPRV | Anti-RVFV | Anti-PPRV | Anti-RVFV |
| RVFV | ||||||||||||||||
| 1 (10 sheep, PPRV 102.5/RVFV 103.5) | 0 | 0 | 100* | 90 | 100 | 100 | 100 | 100 | 100 | 90 | 100 | 100 | 100 | 100 | 100 | 100 |
| 2 (10 sheep. PPRV 103.5/RVFV 104.5) | 0 | 0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 3 (10 sheep, PPRV 104.0/RVFV 104.1) | 0 | 0 | 80 | 20 | 100 | 50 | 100 | 80 | 100 | 90 | 90 | 90 | 90 | 80 | 80 | 90 |
| 4 (10 goats, PPRV 103.3/RVFV 103.2) |
0 | 0 | 100 | 0 | 100 | 33 | 100 | 33 | 100 | 33 | 100 | 41.16 | 100 | 41.16 | 100 | 41.16 |
| 5 (4 sheep, PPRV102.5) | 0 | - | 100 | - | 100 | - | 100 | - | 100 | - | 100 | - | 100 | - | 100 | - |
| 6 (4 goats, PPRV 103.3) | 0 | - | 80 | - | 80 | - | 100 | - | 100 | - | 100 | - | 100 | - | 80 | - |
| 7 (4 sheep, RVFV 103.5) | - | 0 | - | 80 | - | 100 | - | 100 | - | 100 | - | 100 | - | 80 | - | 80 |
| 8 (4 goats RVFV 103.2) | - | 0 | - | 80 | - | 100 | - | 100 | - | 100 | - | 100 | - | 80 | - | 80 |
| 9 and 10 (8 sheep +5 goats) saline placebo (control group) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Note: *Percentage of seroconverted animals in tested groups-=Not Applicable; RVFV: Rift Valley Fever Live Vaccine; PPR: Peste des Petit Ruminants Vaccine
Table 2: Percentage of animals that developed neutralizing antibody following vaccination with monovalent PPRV and RVFV and combined PPRV/RVFV vaccine in 8 groups of sheep and goats, and one group of sheep and one group of goats that received saline as a placebo.
The duration of immunity was evaluated in the group 1 of sheep that received the low doses of combined PPRV/RVFV vaccine. High titers of neutralizing PPRV antibody were maintained reaching 2.46 log10 at twelve-month pv. RVFV Antibody titers peaked on day 28 pv and then the neutralizing antibody titers started to wane reaching a titer of 1.23 log DN50 at 12 months pv (Figure 7). There were no significant differences in antibody titers between the sheep of group 1 and 2 that were vaccinated with the low (102.5 of PPRV/103.5 of RVFV) and high doses (103.5 of PPRV/104.5 RVFV) of vaccine (p>0.01) (Figure 5). On the other hand, the antibody titers for the sheep vaccinated with low and high doses were significantly higher than for sheep vaccinated with the same doses (104 of PPRV/RVFV) (P>0.01).
Figure 7: Average neutralising antibody titer for a period of 12 months of goats and sheep vaccinated with the combined PPRV/RVFV vaccine, respectively.
Serological responses of goats vaccinated with PPRV/RVFV vaccine
The immunogenicity of combined PPRV/RVFV vaccine was evaluated by vaccinating 10 goats SC with 103.3 of PPRV and 103.2 of RVFV (group 4 see Table 1). Also, the immunogenicity of both RVFV and PPRV was determined for sheep vaccinated with monovalent PPRV/RVFV vaccines vs. non vaccinated goats (groups 6 and 10, see Table 1). High titers of PPRV antibody were recorded in vaccinated goats at day 14 pv. In combined vaccine, the peak neutralizing antibody titer was observed on day 14 pv (3.2 log10) vs. 2.9 log10 for the group vaccinated with monovalent PPRV (Figure 6) and all animals had developed PPRV neutralizing antibody (Table 2). For the group of goats that received the combined PPRV/RVFV, antibody against RVFV was detected only in 41.16% vaccinated animals at day 42 pv (Table 2). The maximum antibody titer was observed on day 35 pv with peak neutralizing titers of 3.2 in the goats vaccinated with monovalent RVFV, and on day 21 with titer 1.3 log10 in combined vaccine group, respectively. The titers of neutralizing antibodies in all groups were maintained for a period of 3 months (Figure 7). All unvaccinated controls remained negative indicating an absence of spread of the vaccine virus between the groups of vaccinated and unvaccinated animals (Table 2).
Our results of safety and immunogenicity testing of the PPRV/ RVFV in sheep and goats showed that the vaccine elicited antibody responses with no adverse effects, even with an overdose of the vaccine. Our results demonstrated that the PPRV/RVFV vaccine did not spread from vaccinated to un-vaccinated animals, thus indicating that the viruses did not spread from among the animals.
Discussion
PPR is among the most devastating viral diseases of Small Ruminants (SR) in Africa and Asia causing huge economic losses for small farmers [24]. RVF is a viral disease most seen in domesticated animals such as SR in sub-Saharan Africa [25]. Despite vaccination efforts conducted by many countries, the disease is still enzootic with high prevalence threating more than one billion of SR population throughout the world [26]. In many regions, the low vaccination coverage is probably due to poor infrastructure of SR populations causing difficulty in reaching herds in a large distribution area, especially nomadic or trans-human herds. A global PPR eradication program has been launched by OIE/FAO with the objective to completely eradicate PPR from the world by year 2030 [26].
Combined vaccines present the advantage of reducing vaccination cost in countries where both disease PPR and RVF are enzootic. Encouraging results in the field have been obtained with the combined live sheep goat pox virus (SGPV)-PPR vaccine, which was successfully used for mass vaccination campaigns in Morocco and some African sub-Saharan countries [27,28]. Also, combining PPRV with RVFV will allow for an increase in vaccination coverage for PPR and help to make RVFV vaccine sustainable as a routine vaccination of SR in Africa. Indeed, RVF is a neglected disease during draught or moderate precipitation years and vaccination is applied only after rainy seasons when RVFV emerges to cause epizootics and epidemics [29]. Therefore, the use of a combined RVFV vaccine with PPRV N75 during inter-epizootic periods will establish immunity to both viruses among the SR population that will prevent outbreaks of these devastating diseases in Africa and other regions where these viruses are enzootic.
Our approach for preparing and evaluating combined PPRV and RVFV vaccine was to co-infect Vero cells with both viruses and to separately determine the infectivity titer after 3 serial passages. The results showed slight interference of replication between the viruses. RVFV replication seems to be faster than the PPRV. Replication of PPRV has been documented to be in the cell cytoplasm and does not depend on the function of the nucleus [30]. While RVFV replication also occurs in the cytoplasm, but the viral particle matures in the Golgi apparatus, the targeting of the virus glycoproteins to the Golgi apparatus plays a pivotal role in the virus replication cycle [31]. Our lab recently published in scientific reports, the interference between RVFV and capripoxviruses. Clear interference was detected between RVFV clone 13T and both SPPV and LSDV attenuated viruses. The RVFV attenuated virus interfered with capripoxvirus replication upon their coinfection of cell culture and in vivo when tested in target animals: sheep and cattle (Safini et al, in press).
Based on the promising simultaneous replication of both the PPRV and the RVFV in Vero cells, the safety and immunogenicity of the combined lyophilized PPRV/RVFV live vaccine was tested in sheep and goats, the most affected species by the PPRV and RVFV. This is the first report to our knowledge of this vaccine combination being developed and tested in cells and animals. Vaccine trials done in sheep to determine the appropriate dose of each viral antigen, did not show any difference in the antibody response when the dose was increased for both vaccines.
Sera samples obtained from the vaccinated animals were screened initially for IgG antibody and all positive samples were tested for neutralizing antibody. Overall, the results indicated that all antibody positive animals that tested by the ELISA were also positive for neutralizing antibody.
Vaccinated animals in this study were not challenged to determine if the immune response was protective against an experimental infection with virulent PPRV and RVFV strains. It has been demonstrated for both viruses that the presence of neutralizing antibodies reflect protection against infection and therefore, there is not a need to challenge and cause animal suffering [32,33] Nonetheless, this combined vaccine should be then tested in large field trials.
The combined PPRV/RVFV live vaccine is safe and did not causes any adverse effect after vaccination of highly susceptible animals. Indeed, PPR N75 vaccine strain has been used for decades with no effect on animal health or zootechnic performance. Inoculation of pregnant females was also documented to be safe, no abortion or teratogenic effect. RVF vaccine is however an abortive disease and the vaccine should be tested in pregnant animals at early stages of gestation to confirm safety. The clone 13 attenuated RVFV strain was reported safe in pregnant sheep [34]. However, it was shown that RVFV clone 13 is temperature sensitive with a potential teratogenic effect among pregnant sheep based on experimental studies [35]. The thermostable RVFV C13T strain used in this study was reported to be safe with no evidence of abortions or teratogenicity among the off-springs of pregnant camels. For PPRV, many reports agreed on the safety of PPR vaccine in pregnant animals. Moreover, these studies have shown that upon vaccination, the protective antibodies were seen in 100% of the does and the PPR N75 vaccine effectiveness in inducing seroconversion in pregnant small ruminants by the end of the first week [36,38].
Conclusion
We conclude that the combined live PPRV/RVFV vaccine can be used as a one-shot 102.5 and 103.5 dose safe vaccination of sheep and goats to test lower dose for PPRV to avoid the immunosuppression due to PPRV for large vaccination campaign to prevent both diseases in enzootic country. The vaccine does not require booster and a yearly vaccination of newly recruited susceptible animals into the herds will be sufficient to establish and sustain immunity among SR populations.
Acknowledgments
All authors have seen and approved the content of this manuscript and have contributed significantly to the work. We gratefully acknowledge the support for this study by the Multi- Chemical Industry, Santé Animale and our sponsor International Development Research Centre (IDRC) in Canada.
Author Contributions
The following authors, N.Safini, and M.ELHarrak contributed substantially to the conception and design of the study, N.Safini, Z.Bamouh, and J. Hamdi to the collection and analysis and interpretation of data and the preparation of the manuscript, and D.M. Watts and KO. Tadlaoui contributed to the preparation of the manuscript and interpretation of the data for the work, and all authors participated in the revision of the manuscript, and manuscript and agreed to be accountable for all aspects of the work and approved the final version of this manuscript. All authors reviewed and complied with the journal policies detailed in the guide for authors.
Funding
The authors declare that this study received funding through MCI Santé Animale from the Livestock Vaccine Innovation Fund. LVIF is supported by the Bill & Melinda Gates Foundation (BMGF), Global Affairs Canada (GAC), and Canada's International Development Research Centre (IDRC). The funder was not involved in the study design, collection, analysis, and interpretation of data, the writing of this article or the decision to submit it for publication.
REFERENCES
- Herrero M, Grace D, Njuki J, Johnson N, Enahoro D, Silvestri S et al. The roles of livestock in developing countries. Animal. 2013;7:3-18.
- Wodajo HD, Gemeda BA, Kinati W, Mulem AA, Eerdewijk AV, Wieland B. Contribution of small ruminants to food security for Ethiopian smallholder farmers. Small Ruminant Research. 2020;184:106064.
- Kumar N, Maherchandani S, Kashyap S, Singh S, Sharma S, Chaubey K et al. Peste des petits ruminants virus infection of small ruminants: A comprehensive review. Virusus. 2014;6;2287-2327.
- Dundon WG, Diallo A, Cattoli G. Peste des petits ruminants in Africa: A review of currently available molecular epidemiological data, 2020. Arch Virol. 2020;165:2147-2163.
- Wright D, Kortekaas J, Bowden TA, Warimwe GM. Rift Valley Fever: Biology and Epidemiology.J Gen Virol. 2019;100:1187-1199.
- Kenawy MA, Abdel-Hamid YM, Beier JC. Rift Valley Fever in Egypt and other African countries: Historical review, recent outbreaks and possibility of disease occurrence in Egypt. Acta Trop. 2018;181:40-49.
- Pepin M, Bouloy M, Bird BH, Kemp A, Paweska J. Rift Valley fever virus (Bunyaviridae: Phlebovirus): An update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet Res. 2010;41:61.
- Amarasinghe GK, Bào Y, Basler CF, Bavari S, Beer M, Bejerman N et al. Taxonomy of the order Mononegavirales: update 2017. Arch Virol. 2017;162:2493-2504.
- Gibbs PJ, Taylor WP, Lawman MJP, Bryant J. Classification of peste des petits ruminants virus as the fourth member of the genus morbillivirus. Intervirology. 1979;11:268-274.
- Abudurexiti A, Adkins S, Alioto D, Alkhovsky SV, Avšič-Županc T, Ballinger MJ et al. Taxonomy of the order Bunyavirales: update 2019. Arch Virol. 2019;164:1949-1965.
- Kroeker AL, Babiuk S, Pickering BS, Richt JA, Wilson WC. Livestock challenge models of rift valley fever for agricultural vaccine testing. Front Vet Sci. 2020;7:238.
- Dungu B, Lubisi BA, Ikegami T. Rift valley fever vaccines: current and future needs. Curr Opin Virol. 2018;29:8-15.
- Diallo A, Minet C, Goff LC, Berhe G, Albina E, Libeau G et al. The threat of peste des petits ruminants: Progress in vaccine development for disease control. Vaccine. 2007:25:5591-5597.
- Global Eradication Programme. Peste Des Petits Ruminants Global Eradication Programme.2016.
- Billecocq A, Vialat P, Bouloy M. Persistent infection of mammalian cells by rift valley fever virus. J Gen Virol 1996;77:3053-3062.
- Muller R, Saluzzo JF, Lopez N, Dreier T, Turell M, Smith Jet al. Characterization of clone 13, a naturally attenuated avirulent isolate of rift valley fever virus, which is altered in the small segment. Am J Trop Med Hyg. 1995; 53:405-411.
- Daouam S, Ghzal F, Naouli Y, Tadlaoui KO, Ennaji MM, Oura C et al. Safety and immunogenecity of a live attenuated Rift Valley fever vaccine (CL13T) in camels. BMC Vet Res. 2016;12:154.
- FAO. Global strategy for the control and eradication of PPR. 2015.
- Muller R, Saluzzo JF, Lopez N, Dreier T, Turell M, Smith J, et al. Characterization of clone 13, a naturally attenuated avirulent isolate of Rift Valley fever virus, which is altered in the small segment. Am J Trop Med Hyg. 1995; 53:405-411.
- Daouam S, Fakri FZ, Ennaji MM, El Arkam A, Tadlaoui KO, Oura C et al. Heat stability of the rift valley fever virus clone 13 live vaccines. Trials Vaccinol. 2014;3:61-64.
- Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. American Journal of Epidemiology. 1938; 27:493-497.
- Batten CA, Banyard AC, King DP, Henstock MR, Edwards L, Sanders A et al. A real time RT-PCR assay for the specific detection of peste des petits ruminants virus. Journal of Virological Methods. 2011;171:401-404.
- Busquets N, Xavier F, Martín-Folgar R, Lorenzo G, Galindo-Cardiel I, del Val BP et al. Experimental infection of young adult European breed sheep with rift valley fever virus field isolates. Vector-Borne and Zoonotic Diseases. 2010;10:689-696.
- Mantip SE, Shamaki D, Farougou S. Peste des petits ruminants in Africa: Meta-analysis of the virus isolation in molecular epidemiology studies. J Vet Res. 2019;86:1677.
- Kusiluka L, Kambarage D. Diseasesof smallruminants: A handbook common diseases of sheep and goats in Sub-Saharan Africa. 1996;9:73-78.
- Balamurugan V, Krishnamoorthy P, Raju DSN, Rajak KK, Bhanuprakash V, Pandey AB et al. Prevalence of Peste-des-petits-ruminant virus antibodies in cattle, buffaloes, sheep and goats in India. VirusDisease. 2014;25:85-90.
- Fakri F, Ghzal F, Daouam S, Elarkam A, Douieb L, Zouheir Y et al. Development and field application of a new combined vaccine against Peste des Petits Ruminants and Sheep Pox. Trials Vaccinol. 2015;4:33-37.
- Fakri FZ. Large mass vaccination of small ruminants against Peste des Petits Ruminants and Sheeppox using a combined live attenuated vaccine. J Vet Med Res. 2020;7:1200.
- Gachohi JM, Njenga MK, Kitala P, Bett B. Modelling vaccination strategies against rift valley fever in livestock in Kenya. PLoS Negl Trop Dis. 2017;10: e0005316.
- Taranov D, Yershebulov Z, Amanova Z, Bulatov Y, Barakbayev K, Abduraimov Y, et al. Study of cultural characteristics and interference of peste des petit ruminants virus and sheep pox virus in co-culture. Life Sci J. 2014;11
- Gerrard SR, Nichol ST. Characterization of the golgi retention motif of rift valley fever virus GN glycoprotein. J Virol. 2002;76:12200-12210.
- Zahur AB, Irshad H, Ullah A, Afzal M, Latif A, Ullah RW et al M. Peste des petits ruminants vaccine (Nigerian Strain 75/1) confers protection for at least 3 years in sheep and goats. J Biosci Med (Irvine). 2014;2:27-33.
- Lagerqvist N, Näslund J, Lundkvist A, Bouloy M, Ahlm C, Bucht G. Characterisation of immune responses and protective efficacy in mice after immunisation with Rift Valley Fever virus cDNA constructs. Virology Journal. 2009;6:1-6.
- Dungu B, Louw I, Lubisi A, Hunter P, Teichman BF, Bouloy M. Evaluation of the efficacy and safety of the rift valley fever clone 13 vaccine in sheep. Vaccine. 2010;28:4581-4587.
- Makoschey B, Kilsdonk EV, Hubers WR, Vrijenhoek MP, Smit M, Wichgers Schreur PJ et al V. Rift valley fever vaccine virus clone 13 is able to cross the ovine placental barrier associated with foetal infections, malformations, and stillbirths. Sang RC, editor. PLoS Negl Trop Dis. 2016; 10:e0004550.
- Bodjo S, Couacy-Hymann E, Koffi M, Danho T. Assessment of the duration of maternal antibodies specific to the homologous peste des petits ruminant vaccine “Nigeria 75/1" in Djallonké lambs. Biokemistri. 2006;18:99-103
- Awa DN, Ngagnou A, Tefiang E, Yaya D, Njoya A. Post vaccination and colostral peste des petits ruminants antibody dynamics in research flocks of Kirdi goats and Foulbe sheep of north Cameroon. Prev Vet Med. 2002;55:265-271.
- Markus TP, Adamu J, Kazeem HM, Olaolu OS, Woma TY. Assessment of Peste des petits ruminants antibodies in vaccinated pregnant Kano brown does from Nigeria and subsequent maternal immunity in their kids. Small Ruminant Research. 2019;174:53-56.
Citation: Safinia N, Bamouha Z, Hamidiaa J, Tadlaouia KO, Watts DM, Harraka ME (2021) Evaluation of a Combined Peste des Petits Ruminants and Rift Valley Fever Live Vaccine in Sheep and Goats. J Vaccines Vaccin. S14:003.
Copyright: © 2021 Safini N, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


