PMC/PubMed Indexed Articles
Indexed In
- Online Access to Research in the Environment (OARE)
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Scimago
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 12, Issue 6
Effect of Growth Temperature on Muscle Lipid Class and Fatty Acid Composition in Adult Steelhead Trout (Oncorhynchus mykiss) Fed Commercial Diets with Different ω6 to ω3 Fatty Acid Ratios
Wijekoon M1, Parrish CC1* and Mansour A22Fisheries and Oceans Canada, Northwest Atlantic Fisheries Centre, 80 East White Hills Rd., St. John’s, NL, A1C 5X1, Canada
Received: 10-May-2021 Published: 25-Jun-2021, DOI: 10.35248/2155-9546.21.12.643
Abstract
The effect of fluctuating temperature on muscle lipid composition was examined in 1.8 Kg steelhead trout fed 3 commercial diets with varying proportions (16.5 - 31% total) of Σω3 fatty acids and large differences (5×) in ω6:ω3 fatty acid ratios. Temperature was increased from 13.5°C to 18.0°C and then dropped back down to 13.5°C over a period of 12 weeks. Diet had no significant effect on growth or on total polyunsaturated fatty acid (PUFA) proportions in muscle tissue, but significant changes were observed in individual fatty acids. There was a greater response to changes in diet in the fatty acids than there was to changes in temperature. Muscle C20–C22 ω3PUFA and C18 unsaturated fatty acids reflected dietary inputs leading to very different (up to 40%) ω6:ω3 fatty acid ratios. Independent of diet, increasing temperature significantly increased total lipids and saturated fatty acids, especially 16:0, and decreased monounsaturated ones, especially 18:1ω9, as well as the PUFA 18:2ω6. The ω6:ω3 fatty acid ratio was also lowest at 18.0°C. Increasing total lipids, sterols and saturated fatty acids with increasing temperature, has important implications for both cultured and wild fish in terms of their nutrition, food quality for humans, and resource availability for aquafeeds.
Keywords
Steelhead trout; Fatty acids; Lipid classes; Temperature; ω6:ω3 fatty acid ratio
Introduction
Many studies have investigated the limitations and cost benefits of replacing fish oil (FO) with alternative lipid sources for finfish such as vegetable oils (VO) [1-3] and rendered animal fats (AF) from the beef, pork and poultry industries [4,5]. Studies in the past have focused on growth, health or condition in salmonids [2,4-8], flesh quality [9], fatty acid composition [8,10,11], physical characteristics of the fillet [12,13], metabolic disorders [14] and disease resistance [15,16]. However, less attention has been paid to environmental stresses such as fluctuating water temperature and its effect on fatty acid composition of the final product.
While FO is rich in ω3 long-chain polyunsaturated fatty acids (PUFA) such as DHA (22:6ω3) and EPA (20:5ω3), VO contain C18 ω6, ω9 and ω3 unsaturated FAs, mainly 18:2ω6 and 18:1ω9, and usually high ω6 to ω3 fatty acid ratios [17]. The other alternative, AF are rich in saturated fatty acids (SFA) and monounsaturated fatty acids (MUFA). Although AF may contain trace levels of ω3 PUFA depending on dietary history, species and age, it is not a significant source for FO replacement [4,5].
Salmonids deposit excess dietary lipids in the form of triacylglycerol (TAG) in muscles [10] so that the fatty acid content of fillets is highly reflective of diet and lowering dietary ω3 fatty acid content and raising ω6:ω3 ratios has implications for quality. EPA and DHA consumption reduces cardiovascular risk factors through a variety of effects including anti-inflammatory ones [18]. Inflammation, which may be activated or exacerbated by a high ratio of dietary ω6 to ω3 fatty acids, is thought to connect chronic diseases such as cardiovascular disease, obesity and allergic diseases like asthma [19]. A high value of this ratio has also been associated with cognitive decline and the incidence of dementia [23].
The fatty acid composition of fish is also influenced by fluctuating water temperature [21]. Decreasing water temperature tends to increase unsaturated fatty acid proportions with a corresponding decrease in SFA [21] especially in polar lipids [21-23] as part of homeoviscous adaptation to maintain fluidity. Studies examining the influence of water temperature on fatty acid composition in tissues have usually kept the temperatures at two distinct levels [24-29]. Studies examining multiple changes in water temperature are fewer [30].
Intensive sea cage aquaculture in the North West Atlantic is challenged by uncontrollable and unexpected environmental changes that can be stressful for caged fish, compromising growth and survival, and potentially affecting the quality of the final product for the consumer. Steelhead trout (Oncorhynchus mykiss) is one of the species cultured in the Bay d’Espoir region, Newfoundland that experiences such stressful conditions. This laboratory study was conducted to examine the effect of temperature fluctuations on muscle lipid and fatty acid composition in adult steelhead trout raised on FO substituted diets. They were fed three commercial diets that have been used by the aquaculture industry in Canada. These diets were manufactured with either FO as the primary source of lipids or with FO partially substituted with AF or VO. Water temperature was manipulated to mirror changes occurring naturally during the growth season and the effect of dietary lipid composition on muscle lipid class and fatty acid composition was examined. A parallel study on liver membrane properties was conducted [29].
The maximum temperature difference examined in this study is 4.5°C which is as high as might be expected in cold seasons in certain regions with global warming of 1.5°C above pre-industrial levels [31]. This study provides insight into the effects of such a change on fish lipid composition with implications not only for fish nutrition and the product for the consumer, but for the quality of resources available for aquafeeds. One of the biggest challenges facing the future world aquaculture industry is access to quality ingredients for aquafeeds [32].
Materials and Methods
Experimental fish
This study was conducted at the Ocean Sciences Centre (OSC), Memorial University of Newfoundland (MUN), St. John’s, with the approval of MUN’s Animal Care Committee. Three hundred and seventy Steelhead trout averaging 1.6 Kg body weight each were transported to the OSC from a Bay d’Espoir aquaculture site. The condition of the fish was observed visually during the journey and dissolved oxygen (O2) and water temperature were measured during transportation and were within acceptable limits.
At the OSC, fish were moved to a 45 m3 holding tank at ambient temperature (6 ± 1°C). Fish were examined by a veterinarian at the end of the 1st week to determine any stress related health problems. Samples obtained during the diagnostic visit did not reveal any significant infectious disease in the population. Additional O2 was also provided during the first week at a rate of 0.25 – 0.5 L min-1 to minimize transport stress. Dissolved O2 and temperature was monitored twice daily and dead fish were promptly removed and examined for external lesions. All waste accumulated at the bottom of the tank was removed daily.
Feed was introduced gradually to alleviate transportation stress. Appetite improved after 1 - 2 weeks, after which they were fed twice a day, morning and evening. During the first month, fish were given the same feed type used at the aquaculture site (Corey feed, Corey Nutrition Company, Fredericton, NB, Canada). However, they were switched to the experimental base diet (M-ω3: section 2.3) at the end of first month. Fish were held in the holding tank for approximately 3-4 months prior to the experiment.
Experimental tanks
Six identical 6000 L tanks were used for the experiment. Fiftyfive fish were randomly picked and distributed into each of the experimental tanks. Fish were held at ambient water temperature (12 to 13°C) with a water flow of 6 -7 L min-1 for acclimation. The system was flow-through from Logy Bay, and lighting was automatically controlled to follow the daily light/dark cycles. Dissolved O2, salinity and temperature were monitored daily.
Experimental feed
Three different commercially available diets were used based on information given on raw materials used by the manufacturers. On receipt, the diets were analyzed to determine lipid class and fatty acid composition. The three diets were named according to the relative proportions of ω3 fatty acids as H-ω3 (higher ω3), M-ω3 (medium ω3), and L-ω3 (lower ω3) (Table 1), based on the lipid analysis (Table 2). According to the feed manufacturers (personal communication) the protein and fat content of the H-ω3 diet mainly originated from fish meal and FO. In contrast, in the M-ω3 and L-ω3 diets, fish meal and FO were partially replaced by a combination of terrestrial AF and VOs. All feeds were stored in a -20°C freezer to minimize lipid oxidation. A portion of each feed was kept separately in a walk-in cooler for daily use. All fish were fed with the experimental base diet (M-ω3) during the acclimation period.
| Composition | H-ω3 | M-ω3 | L-ω3 |
|---|---|---|---|
| Crude protein (min) % | 41 | 42 | 42 |
| Crude fat (min) % | 24 | 27 | 23 |
| Crude fibre (max) % | 1.5 | 4 | 4 |
| Calcium (actual) % | 1.6 | 1.1 | 0.9 |
| Phosphorus (actual) % | 1.3 | 1 | 0.8 |
| Sodium (actual) % | 0.5 | 0.4 | 0.54 |
| Vitamin A (min) IU/Kg | 5000 | 5000 | 5000 |
| Vitamin D (min) IU/Kg | 2400 | 4000 | 4000 |
| Vitamin E (min) IU/Kg | 200 | 250 | 250 |
Table 1: Label information for the 3 commercial diets used in the experiment. The three diets were identified by the level of ω3 polyunsaturated fatty acids (PUFA) determined to be present in the diet (Higher, H-ω3; Medium, M-ω3; Lower, L-ω3).
| H-ω3 | M-ω3 | L-ω3 | |
|---|---|---|---|
| Total lipid (ww-1 %) | 34.0 ± 6.42 | 33.8 ± 1.48 | 29.5 ± 5.27 |
| Neutral lipid (ww-1 %) | 31.7 ± 6.3 | 32.0 ± 1.27 | 27.8 ± 5.04 |
| Polar lipid (ww-1 %) | 2.26 ± 0.12 | 1.76 ± 0.31 | 1.74 ± 0.25 |
| Lipid class (% total lipid)a | |||
| Triacylglycerol | 91.6 ± 0.88 | 88.9 ± 3.12 | 89.9 ± 0.71 |
| Sterol | 0.77 ± 0.18a | 5.62 ± 2.77b | 3.77 ± 1.06b |
| Phospholipid | 3.86 ± 0.52 | 3.55 ± 0.37 | 3.98 ± 0.58 |
| Fatty acid (% total fatty acid) | |||
| 14:00 | 5.96 ± 0.05a | 4.6 ± 0.08b | 3.63 ± 0.03c |
| 16:00 | 15.5 ± 0.09a | 19.0 ± 0.3b | 19.7 ± 0.07c |
| 18:00 | 2.67 ± 0.02a | 6.18 ± 0.18b | 6.86 ± 0.07c |
| 18:1ω9 | 8.8 ± 0.14a | 21.0 ± 0.42b | 23.3 ± 0.24c |
| 20:1ω9 | 4.8 ± 0.27a | 1.45 ± 0.04b | 2.13 ± 0.03c |
| 22:1ω11(13) | 7.57 ± 0.46a | 1.68 ± 0.08b | 2.53 ± 0.06c |
| 18:2ω6 | 2.49 ± 0.13a | 8.07 ± 0.17b | 9.8 ± 0.21c |
| 18:3ω3 | 0.7 ± 0.0a | 0.75 ± 0.04a | 0.94 ± 0.03b |
| 20:4ω6 (ARA) | 0.9 ± 0.01a | 0.88 ± 0.01a | 0.78 ± 0.0b |
| 20:5ω3 (EPA) | 15.7 ± 0.28a | 10.5 ± 0.51b | 8.34 ± 0.1c |
| 22:5ω3 (DPA) | 2.36 ± 0.01a | 1.28 ± 0.06b | 1.22 ± 0.03b |
| 22:6ω3 (DHA) | 9.26 ± 0.28a | 6.27 ± 0.21b | 4.42 ± 0.08c |
| ∑ SFAb | 25.4 ± 0.09a | 30.9 ± 0.35b | 31.3 ± 0.05b |
| ∑ MUFAc | 33.4 ± 0.79a | 34.0 ± 0.21a | 37.4 ± 0.33b |
| ∑ PUFAd | 40.0 ± 0.72a | 34.3 ± 0.57b | 30.5 ± 0.39c |
| P:S | 1.57 ± 0.02a | 1.11 ± 0.03b | 0.98 ± 0.01c |
| ∑ω 3 | 31.1 ± 0.56a | 21.0 ± 0.67b | 16.5 ± 0.24c |
| ω 6: ω 3 | 0.13 ± 0.003a | 0.47 ± 0.02b | 0.69 ± 0.009c |
| ∑ Terrestriale | 3.19 ± 0.13a | 8.83 ± 0.21b | 10.7 ± 0.24c |
| ∑ Marinef | 27.3 ± 0.54a | 18.1 ± 0.66b | 13.9 ± 0.2c |
Diets labelled as: H-ω3 (Higher ω3 fatty acids), M-ω3 (Medium ω3 fatty acids) and L-ω3 (Lower ω3 fatty acids).
Saturated fatty acids (SFA), Monounsaturated fatty acids (MUFA), Polyunsaturated fatty acids (PUFA), polyunsaturated to saturate ratio (P:S), Omega (ω), Arachidonic acid (AA), Eicosapentaenoic acid (EPA), Docosahexaenoic acid (DHA), Docosapentaenoic acid (DPA).
aLipid class (% total lipid), which also includes: hydrocarbon, steryl/wax ester, acetone mobile polar lipids at <2% each.
bSum of saturated fatty acids (SFA), which also includes: i15:0, 15:0, ai16:0, i17:0, ai17:0, 20:0, 22:0 and 23:0 at < 1.0% each.
cSum of monounsaturated fatty acids (MUFA), which also includes: 14:1, 15:1, 16:1ω7, 16:1ω9, 16:1ω5, 17:1, 18:1ω7, 20:1ω11, 20:1ω9, 22:1ω11, 22:1ω9 and 24:1.
dSum of polyunsaturated fatty acids (PUFA), which also includes: 16:2ω4, 16:3ω4, 16:4ω3, 16:4ω1, 18:2ω4, 18:3ω6, 18:4ω1, 18:4ω3, 20:2a, 20:2ω6, 20:3ω3, 21:5ω3 and 22:4 ω6 at < 1.0% each.
eTerrestrial fatty acids: 18:2ω6 and 18:3ω3.
fMarine fatty acids: EPA, DPA and DHA
Table 2: Selected lipid class (%), total lipid, polar lipid, neutral lipid (% ww-1) and fatty acid (%) average composition (mean ± SD) and ratios of commercial feed containing higher ω3 polyunsaturated fatty acids (H-ω3), medium ω3 polyunsaturated fatty acids (M-ω3) and lower ω3 polyunsaturated fatty acids (L-ω3).
Two tanks were randomly assigned for each feed by drawing a ballot. Fish were fed twice daily to satiation and leftover feed was measured to calculate the amount consumed. The point of satiation was determined as follows: a known amount of feed (usually 1- 2% of body weight) was measured in advance for each tank. A handful of feed pellets was thrown into the tank and observed for consumption. This was continued until more than half the pellets thrown in sank to the bottom of the tank uneaten. Once this point was reached, another handful of feed was thrown into the tank after 5-10 min. If left uneaten, this was considered the satiation point.
Experimental temperature
Fish were left for almost 2 weeks at 13.0 ± 1.0°C to acclimatize to the experimental tanks. Then average temperature was increased, following seasonal temperature changes, in a stepwise manner from 13.5°C (1st sample set) to plateau at 16.5°C (2nd sample set), then up to a maximum of 18.0°C (3rd sample set: Figure 1). Thereafter, water temperature was dropped from 18.0°C back to 13.5°C (5th sample set) with the same in-between step at 16.5°C (4th sample set). At each step the temperature was increased or decreased gradually to the next level over a 2 - 3 day period and left stable thereafter for 14 ± 1 days. Ambient water was heated and adjusted through an automatically controlled system at the header tank to obtain the required temperatures in the experimental tanks.
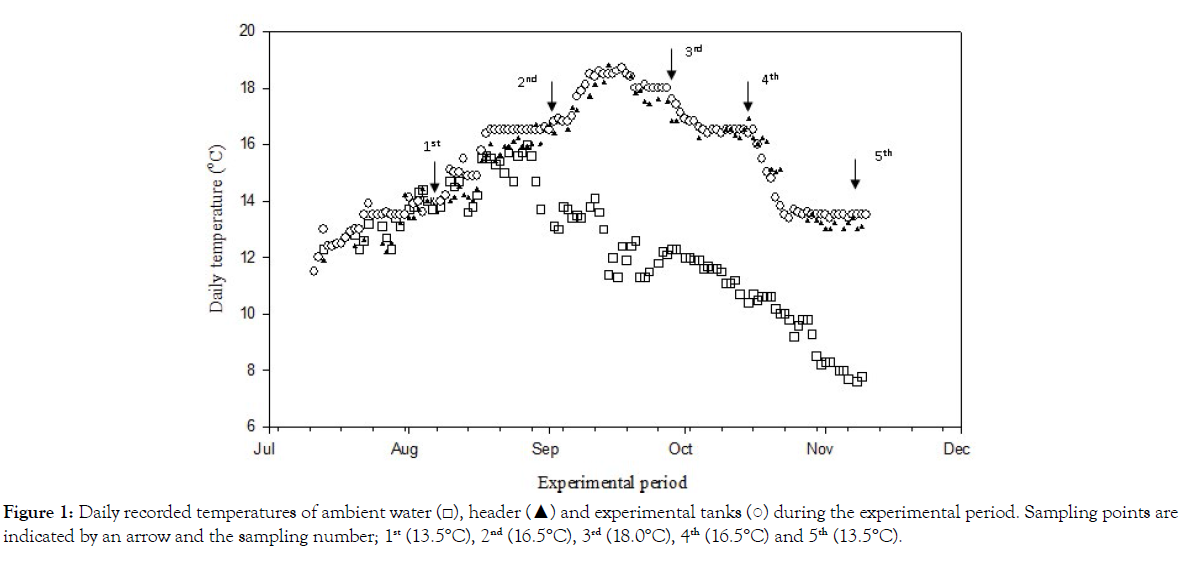
Figure 1: Daily recorded temperatures of ambient water (□), header (▲) and experimental tanks (○) during the experimental period. Sampling points are indicated by an arrow and the sampling number; 1st (13.5°C), 2nd (16.5°C), 3rd (18.0°C), 4th (16.5°C) and 5th (13.5°C).
Specific growth rate and food conversion efficiency
The initial mean wet weight of adult steelhead trout assigned to H-ω3 was 1662 ± 319 g, to M-ω3 it was 1741 ± 302 g, and to L-ω3 it was 1945 ± 721 g. Specific growth rate (SGR) and food conversion efficiency (FCE) was calculated for fish fed the 3 diets for each sampling period.
Sampling protocol
At the end of the acclimation period prior to the feeding trial, fish were fed with the previously assigned feed types (see above) for a 12 week period. However, fish were deprived of two feedings prior to sampling. A minimum of six fish per treatment were lethally sampled at the end of each thermal period with an overdose of the anaesthetic tricaine methane sulfonate (TMS, MS-222). Muscle samples for lipid class and fatty acid analysis were obtained from the left epaxial region of the fish caudo dorsal to the pectoral and ventral to the anterior base of the dorsal fin. Samples of muscle weighing approximately 1 g were collected in 50 ml glass vials previously cleaned for lipid residues as described below. Then each vial was flushed with nitrogen (N2) after filling with 4 ml CHCl3 and sealed with Teflon lined caps and Teflon tape and stored at -20°C.
Lipid extraction and analysis
Lipid extraction
All material coming into contact with the samples for lipid analysis, was made of either glass or was Teflon coated. All glassware was heated in a muffle furnace at 450°C for 12 hrs and lipid cleaned by rinsing three times with MeOH followed by three times with CHCl3. All solvents were of analytical or chromatographic grade. Distilled water used for experimental analysis was washed three times with CHCl3 in a separatory funnel to remove lipid residues.
Total lipids were extracted from samples in CHCl3/MeOH following Parrish [33] using a modified Folch procedure [34] in which samples were homogenized on ice (Brinkman Polytron blender, NY) in 2:1 chloroform:methanol and then with chloroform-extracted water to a final dilution ratio of 2:1:1. They were then sonicated in ice for 4 min and centrifuged for 3 min (3000 g). The bottom, organic layer was then removed by double pipetting.
Lipid class separation
Lipid classes were separated using thin layer chromatography with flame ionization detection in a MARK V Iatroscan (Iatroscan Laboratories, Tokyo, Japan). Specific amounts of lipid classes contained in each sample were determined by spotting lipid extracts on silica gel-coated rods and separating with a three-stage development system: 99:1:0.05 hexane/diethyl ether/formic acid, 80:20:1 hexane/diethyl ether/formic acid, acetone and 5:4:1 chloroform/methanol/water [35]. At the end of each development system with different polarities, the rods were partially scanned to detect lipid classes. The flow of the FID combustion gasses, air and hydrogen were set to 200 ml min-1 and 20 ml min-1 respectively. The resulting three chromatograms were combined using T-data scan software (RSS, Bemis, Tennessee, USA). Lipid classes were identified and quantified using standards: n-nonadecane, cholesteryl palmitate, 3-hexadecanone, tripalmitin, palmitic acid, 1-hexadecanol, cholesterol, 1-monopalmitoyl-rac-glycerol, and dipalmitoyl phosphatidylcholine (Sigma-Aldrich Corp., Oakville, Ontario, Canada).
Fatty acid analysis
The same crude lipid extracts were also subjected to transesterification using 14% boron trifluoride in MeOH to produce fatty acid methyl esters (FAME) following a procedure based on [29] as outlined in [34]. Analysis of FAME derivatives was conducted using an HP 6890 model gas chromatograph (GC) equipped with an FID and an HP 7683 autosampler (Agilent Technologies Canada Inc., Mississauga, Ontario, Canada). Fatty acid peaks were integrated using HP Enhanced Chemstation G 1701BA Version B.OO.OO (Agilent Technologies Canada Inc., Mississauga, Ontario, Canada) and identified against known standards (PUFA 1, PUFA 3, BAME, and a Supelco 37 component FAME mixture, Sigma-Aldrich Canada Ltd, Oakville, Ontario, Canada).
Statistical analyses
All data sets were examined to verify normality, independence and homogeneity of variance before further analysis was undertaken. Muscle and feed lipid class and fatty acid data were subjected to analysis of variance using the General Linear Model procedure of the Statistical Analysis System (GLM procedure, SPSS 13.0 for Windows). The test for muscle lipid classes and fatty acids was performed with temperature and feed type as explanatory variables (two-way ANOVA) with interactions (feed x temperature) to determine the effect of temperature and feed type on lipid class and fatty acid composition. If significant interactions were present the effect of feed type was examined at each sampling temperatures using feed type as the explanatory variable. Multiple comparisons of means for lipid classes and fatty acids were made using Tukey corrections. Pearson correlation analyses were performed using Minitab 16 (Statistical Software, State College, PA, USA), and multivariate statistics were run in PRIMER (Plymouth Routines in Multivariate Ecological Research; PRIMER-E Ltd, version 6.1.15, Ivybridge, UK) with the PERMANOVA+ add-on. Significance was set at α = 0.05 for all tests.
Results
Varying growth temperature effects on muscle lipid class and fatty acid composition were examined in 1.8 Kg steelhead trout fed commercial diets with varying total ω3 fatty acids and large differences in ω6:ω3 ratios. Temperature was increased in a stepwise manner from 13.5°C with plateaus to a maximum of 18.0°C following ambient temperature Figure 1. Thereafter, temperature was dropped from 18.0°C back to 13.5°C. Dissolved oxygen was maintained at 104.0 ± 8.0% on average.
Feed lipid class and fatty acid composition
Label information for the three feeds used in the experiment is given in Table 1. To preserve anonymity, feeds were identified by the level of ω3 as H-ω3, M-ω3 or L-ω3. Total lipid (TL), neutral lipid (NL) and polar lipids (Polar) (ww-1%) were not significantly different among the three feed types (Table 2). The most abundant classes of lipid in the three feed types were triacylglycerol (TAG), sterol (ST), and phospholipid (PL). The H-ω3 feed had a lower ST than both M-ω3 and L-ω3 (p ≤ 0.031) feed. TAG had the highest proportion and it was not significantly different among the feeds. Similarly PL was not significantly different among feeds (Table 2).
Individual and total MUFA, SFA, PUFA in the feeds are given in Table 2. Both ΣPUFA and Σω3 proportions were significantly different (p<0.001) among the feeds with H-ω3 having the highest and L-ω3 diet having the lowest levels (Table 2). SFA was lowest in fish oil rich H-ω3 (p<0.001) feed, and MUFA was highest (p<0.001) in vegetable oil rich L-ω3 feed (Table 2). The PUFA:SFA (P:S) ratio was highest in H-ω3 feed and lowest in L-ω3 feed (p<0.001). Individual fatty acids were present in significantly different proportions in each feed (p ≤ 0.026) except 22:5ω3 in M-ω3 and L-ω3 and 18:3ω3 and 20:4ω6 in H-ω3 and M-ω3. Terrestrial (18:2ω6) and typical marine origin ω3 fatty acids (EPA, DPA and DHA) showed the greatest differences among PUFA in the three feed types (Table 2) leading to large differences in ω6:ω3 ratios: H-ω3 had the lowest ω6:ω3 ratio and L-ω3 the highest. Marine origin fatty acids were lowest in L-ω3 diets and typical terrestrial plant fatty acids (18:2ω6 and 18:3ω3) were highest in L-ω3 diets.
Growth performance
Temperature and feed had no significant effect on growth of fish or mortality during the experimental period. The average wet weight of the fish increased 30 – 40% from 1.79 ± 0.40 kg to final weights of 2.63 ± 0.20 kg for fish fed H-ω3, 2.27 ± 0.31 kg for M-ω3, and 2.37 ± 0.25 kg for L-ω3.
Tissue lipid class composition
Table 3 shows the effect of diet on lipid class composition (%) in muscle tissue of Steelhead trout fed the three diets. Both TL and NL (ww-1%) in muscle tissue were affected by the increase in temperature with a significant increase in L-ω3 fed fish at 18.0°C (p ≤ 0.03). The difference in total lipid was also seen on decreasing to 16.5°C. Overall the total lipid concentration was positively correlated with temperature (r=0.622, p=0.023).
| Sample | 1 | 2 | 3 | 4 | 5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp | 13.5°C | 16.5°C | 18.0°C | 16.5°C | 13.5°C | ||||||||
| Feed | M-ω3 | H-ω3 | M-ω3 | L-ω3 | H-ω3 | M-ω3 | L-ω3 | H-ω3 | M-ω3 | L-ω3 | H-ω3 | M-ω3 | L-ω3 |
| 14:00 | 3.58 ± 0.4** | 3.2 ± 0.4 | 3.0 ± 0.2 | 3.2 ± 0.3 | 3.5 ± 0.4 | 3.2 ± 0.3 | 2.9 ± 0.3 | 2.8 ± 0.5 | 2.8 ± 0.3* | 3.0 ± 0.3 | 3.4 ± 0.3a | 2.7 ± 0.2b* | 2.8 ± 0.1b |
| 16:00 | 15.4 ± 0.8 | 14.8 ± 1.1 | 15.1 ± 0.5 | 15.1 ± 0.6§ | 15.8 ± 0.6 | 17.3 ± 0.6** | 16.8 ± 1.7§ | 15.3 ± 1.6 | 15.5 ± 1.9 | 15.6 ± 1.2§ | 14.7 ± 1.0 | 14.1 ± 2.7* | 14.5 ± 0.8§§ |
| 18:00 | 3.6 ± 0.3** | 3.8 ± 0.3 | 3.9 ± 0.1 | 3.8 ± 0.1 | 3.9 ± 0.1a | 4.8 ± 0.7b* | 4.4 ± 0.4ab | 3.3 ± 1.7 | 4.1 ± 0.4 | 4.41 ± 0.8 | 3.7 ± 0.2 | 4.0 ± 1.0 | 3.78 ± 0.2 |
| ∑ SFAa | 23.5 ± 1.3 | 22.6 ± 1.6 | 22.9 ± 0.8 | 23.0 ± 0.9 | 24.1 ± 0.9 | 26.6 ± 1.6** | 24.8 ± 2.3 | 22.2 ± 2.2 | 23.3 ± 2.4 | 23.9 ± 2.0 | 22.6 ± 1.5 | 21.0 ± 3.1* | 21.7 ± 1.0 |
| 18:1ω9 | 28.0 ± 1.6 | 27.9 ± 1.6 | 27.9 ± 1.1 | 27.7 ± 1.3§ | 24.5 ± 1.5 | 25.2 ± 2.8** | 26.3 ± 2.8§ | 23.0 ± 4.2 | 26.3 ± 3.6 | 26.1 ± 1.5§ | 25.7 ± 2.7a | 29.9 ± 3.4b* | 29.7 ± 1.5b§§ |
| 22:1ω11 | 1.0 ± 0.2 | 1.4 ± 0.3 | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.6 ± 0.5a | 0.85 ± 0.3b | 1.0 ± 0.2b | 1.0 ± 0.4 | 0.9 ± 0.2 | 0. 9 ± 0.2 | 1.53 ± 0.6a | 0. 9 ± 0.1b | 1.0 ± 0.1 |
| ∑ MUFAb | 43.6 ± 1.9 | 43.6 ± 1.7 | 43.0 ± 1.4 | 43.2 ± 1.6 | 42.4 ± 2.5 | 40.4 ± 2.3 | 42.4 ± 3.2 | 39.2 ± 6.01 | 41.3 ± 5.2 | 43.1 ± 0.8 | 43.1 ± 2.2 | 45.1 ± 3.5 | 46.0 ± 1.5 |
| 18:2ω6 | 9.5 ± 0.6** | 9.1 ± 0.6 | 9.4 ± 0.1 | 9.4 ± 0.5 | 7.0 ± 0.8a | 8.1 ± 0.7* | 8.9 ± 0.8b | 8.0 ± 0.8 | 9.1 ± 0.9 | 9.3 ± 0.6 | 8.2 ± 1.0a | 9.5 ± 1.1b** | 9.7 ± 0.2b |
| 20:4ω6 | 0.7 ± 0.07 | 0.69 ± 0.1 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.1 | 1.1 ± 1.3 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.61 ± 0.1 | 0.7 ± 0.1 | 0.5 ± 0.2 | 0.6 ± 0.1 |
| 20:5ω3 | 4.4 ± 0.7 | 4.5 ± 0.6 | 4.4 ± 0.4 | 4.9 ± 0.6 | 5.2 ± 0.3 | 4.0 ± 1.8 | 4.2 ± 0.5 | 5.6 ± 0.9a | 4.7 ± 0.6 | 4.3 ± 0.4b | 5.1 ± 0.5a | 4.06 ± 0.4b | 4.1 ± 0.5b |
| 22:5ω3 | 1.8 ± 0.1 | 2.1 ± 0.1 | 2.0 ± 0.3a | 2.4 ± 0.1b | 2.3 ± 0.2 | 1.7 ± 0.7 | 2.1 ± 0.6 | 2.6 ± 0.2a | 2.2 ± 0.2b | 2.2 ± 0.2 | 2.5 ± 0.1 | 2.2 ± 0.4 | 2.2 ± 0.1 |
| 22:6ω3 | 9.8 ± 1.0 | 11.0 ± 0.8 | 11.1 ± 1. | 9.9 ± 0.6 | 11.8 ± 0.4 | 11.8 ± 2.4 | 10.7 ± 2.7 | 16.2 ± 4.2a | 12.9 ± 3.3 | 10.7 ± 1.8b | 11.3 ± 1.6 | 10.5 ± 1.4 | 9.5 ± 1.5 |
| ∑ PUFAc | 32.1 ± 1.8 | 33.3 ± 1.5 | 33.5 ± 1.5 | 33.2 ± 1.3 | 32.9 ± 1.8 | 32.4 ± 2.7 | 32.2 ± 2.3 | 38.1 ± 4.2 | 34.9 ± 3.6 | 32.4 ± 2.5 | 33.8 ± 2.0 | 32.2 ± 2.3 | 31.7 ± 2.2 |
| P:S | 1.4 ± 0.1 | 1.5 ± 0.1+ | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1+ | 1.2 ± 0.1 | 1.31 ± 0.1 | 1.7 ± 0.1a++ | 1.51 ± 0.1 | 1.37 ± 0.2b | 1.5 ± 0.1 | 1.5 ± 0.3 | 1.5 ± 0.1 |
| ∑ω 3 | 18.6 ± 1.8 | 19.9 ± 1.0+ | 19.9 ± 1.3 | 19.6 ± 1.3 | 21.7 ± 0.6+ | 20.4 ± 2.6 | 19.5 ± 2.8 | 26.3 ± 4.8a++ | 21.9 ± 4.0 | 19.3 ± 2.3b | 21.3 ± 2.2a+ | 18.8 ± 1.7 | 18.0 ± 2.1b |
| ω 6: ω 3 | 0.6 ± 0.4 | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.4 ± 0.04a | 0.51 ± 0.1** | 0.58 ± 0.1b | 0.4 ± 0.1a | 0.54 ± 0.1 | 0.6 ± 0.1b | 0.5 ± 0.1a | 0.6 ± 0.1b* | 0.7 ± 0.1b |
| EPA:AA | 6.2 ± 0.7 | 5. 8 ± 1.2 | 5.7 ± 0.7 | 6.6 ± 0.6 | 6.9 ± 0.9 | 5.15 ± 2.3 | 5.74 ± 0.8 | 5.9 ± 0.6 | 5.8± 0.7 | 5.7 ± 0.4 | 6.5 ± 0.9a | 4.9 ± 0.6b | 5.4 ± 0.5b |
| ∑ Terrestrial | 10.4 ± 0.6** | 9.9 ± 0.6 | 10.2 ± 0.2 | 10.2 ± 0.6 | 7.7 ± 0.9a | 8.9 ± 0.8* | 9.7 ± 0.9b | 8.5 ± 0.9a | 9.8 ± 1.0 | 10.0 ± 0.7b | 9.0 ± 1.0a | 10.2 ± 1.2 | 10.5 ± 0.3b |
| ∑ Marinee | 15.9 ± 1.6 | 17.6 ± 0.8+ | 17.4 ± 1.4 | 17.1 ± 1.0 | 19.4 ± 0.5+ | 17.5 ± 2.8 | 17.0 ± 3.0 | 24.4 ± 5.1a++ | 19.8 ± 4.1 | 17.2 ± 2.4b | 18.9 ± 2.2a+ | 16.8 ± 1.7 | 15.7 ± 2.1b |
Significant differences among diets at each temperature and among temperatures for each diet are indicated by superscript letters and by symbols respectively.
Data analyzed by 2-way ANOVA.
aSum of saturated fatty acids (SFA), which also includes: i15:0, 15:0, ai16:0, i17:0, ai17:0, 20:0, 22:0 and 23:0 at < 1.0% each.
bSum of monounsaturated fatty acids (MUFA), which also includes: 14:1, 15:1, 16:1ω7, 16:1ω9, 16:1ω5, 17:1, 18:1ω7, 20:1ω11, 20:1ω9, 22:1ω11, 22:1ω9 and 24:1 at < 1.0% each.
cSum of polyunsaturated fatty acids (PUFA), which also includes: 16:2ω4, 16:3ω4, 16:4ω3, 16:4ω1, 18:2ω4, 18:3ω6, 18:3ω3,18:4ω1, , 20:2a, 20:2ω6, 20:3ω3, 21:5ω3 and 22:4ω6 at < 1.0% each.
dTerrestrial fatty acid: 18:2ω6 and 18:3ω3.
eMarine fatty acids: EPA, DPA and DHA.
Temperature (Temp), Polyunsaturated to saturate ratio (P:S), Omega (ω), Eicosapentaenoic acid (EPA), Docosahexaenoic acid (DHA), Arachidonic acid (AA).
Table 3: Effect of H-ω3, M-ω3 and L-ω3 diet and temperature on muscle fatty acid composition (% total) of steelhead trout (Oncorhynchus mykiss).
Both diet and temperature had significant (p ≤ 0.027) effects on major lipid class composition in muscle tissue (Table 4). Significant differences occurred in TAG, ST, PL and total polar lipid, both at the end of the first temperature increase (2nd sampling point) and after the final drop to 13.5°C (5th sampling point). TAG was the most abundant lipid class (>85%) present in muscle tissue. The PL level was only affected at the end of the temperature trial (13.5°C), where H-ω3 fed fish had a significantly higher total polar lipid concentration over the other two treatments (p < 0.001). ST proportions were significantly higher in L-ω3 fed fish at the 2nd, 3rd and 4th sampling points. However, when the temperature was dropped back at the end of the experiment (5th sampling) ST of M-ω3 was significantly higher than H-ω3. ST in H-ω3 fed fish was at a comparatively lower level compared to with the other two diets throughout.
| Sample | 1 | 2 | 3 | 4 | 5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp | 13.5°C | 16.5°C | 18.0°C | 16.5°C | 13.5°C | ||||||||
| Feed | M-ω3 | H-ω3 | M-ω3 | L-ω3 | H-ω3 | M-ω3 | L-ω3 | H-ω3 | M-ω3 | L-ω3 | H-ω3 | M-ω3 | L-ω3 |
| TAG | 94.3±1.7 | 91.3±0.8a+ | 93.3±2a* | 88.2±1.6b§ | 94.4±1.5+ | 92.8±2.3* | 91.9±3.3§ | 93.8±2.9+ | 89.6±3.2** | 91.5±1.7§ | 84.9±2.8a++ | 93.4±0.7b* | 93.4±2.0b§§ |
| ST | 0.6±0.34 | 0.4±0.1 | 0.4±0.2a* | 0.8±0.1b§ | 0.7±0.3a | 1.7±1.0** | 2.8±1.7b§§ | 0.5±0.1a | 1.1±0.5** | 1.2±0.3b§ | 0.6±0.3a | 1.4±0.7b** | 0.9±0.4§ |
| PL | 2.85±1.3 | 4.6±0.3 | 3.7±1.0 | 4.0±1.0 | 2.1±0.7 | 3.5±2.2 | 3.1±1.4 | 3.1±1.8 | 6.6±4.8 | 6.1±2.4 | 12.1±2.4a | 3.6±0.5b | 2.8±1.7b |
| TL | 13.3±5.28 | 20.4±4.9 | 18.9±10.0 | 14.1±5.0 | 13.7±7.7a | 22.8±9.2a | 23.2±2.9b | 17.3±6.9a | 18.2±4.9 | 20.3±3.0b | 15.4±6.1 | 13.5±3.0 | 15.7±9.1 |
| NL | 12.8±5.2 | 19.2±4.7 | 18.2±10.0 | 13.3±5.0 | 13.2±7.7a | 22.1±8.8a | 22.3±3.0b | 16.9±7.0 | 17.0±4.4 | 18.9±2.7 | 13.4±5.8 | 12.9±3.0 | 15.1±9.1 |
| Polar | 0.52±0.14 | 1.3±0.4a | 0.7±0.2b | 0.8±0.1b | 0.51±0.1 | 0.75±0.4 | 0.9±0.3 | 0.5±0.2 | 1.20±0.9 | 1.4±0.5 | 2.01±0.5a | 0.61±0.1b | 0.5±0.2b |
Significant differences among diets at each temperature and among temperatures for each diet are indicated by superscript letters and by symbols respectively.
Data analyzed by 2-way ANOVA.
Diets labelled as: H-ω3 (Higher ω3 fatty acids), M-ω3 (Medium ω3 fatty acids) and L-ω3 (Lower ω3 fatty acids).
Temperature (Temp), Triacylglycerol (TAG), Sterol (ST), Phospholipid (PL), Total lipid (TL), Neutral lipid (NL), Polar lipid (Polar).
Table 4: Effect of H-ω3, M-ω3 and L-ω3 diets on total lipid, neutral lipid, polar lipid (ww-1%) and percent lipid class composition of muscle tissue of steelhead trout (Oncorhynchus mykiss) at different temperatures.
The effect of temperature on TAG and ST proportions in M-ω3, H-ω3 and L-ω3 fed fish during the experimental period is shown in Table 3 as well. The effect on TAG was prominent at the final temperature of 13.5°C, with H-ω3 fish storing less TAG in muscle compared to those fed the other diets. Decreasing temperature also increased the polar lipid fraction of H-ω3 fed fish in comparison to fish fed the other diets.
The TAG proportions in M-ω3 fed fish significantly decreased with the drop in temperature from 18.0°C to 16.5°C and significantly increased with the further drop in temperature to 13.5°C (p ≤ 0.039). TAG proportions in L-ω3 fed fish had a similar pattern to M-ω3 fed fish without the significant drop in proportion with the drop in temperature from 18.0°C to 16.5°C. The ST proportion of H-ω3 fed fish was not affected by temperature but ST of both M-ω3 and L-ω3 fed fish increased significantly with increasing temperature (p ≤ 0.029).
Tissue fatty acid composition
Fatty acid composition of muscle tissue of fish fed each diet at each experimental temperature is given in Table 4. There was a greater response to changes in diet in the fatty acids than there was to changes in temperature. Fourteen of the 19 variables in Table 4 showed significant differences for diet while only 11 showed significant differences for temperature. The greater response to diet was confirmed by permutational multivariate analysis of variance (PERMANOVA). PERMANOVA shows that overall, the fatty acid profiles Table 4 were significantly different between H-ω3 and L-ω3 fish (p(perm)=0.029). Similarity percentage analysis (SIMPER) shows that EPA+DPA+DHA, Σω3 FAs, and 18:1ω9 were driving the dissimilarity at a cumulative level of 39%.
Among the fatty acid sums and ratios in Table 2 the ω6:ω3 fatty acid ratio showed the greatest difference being 5 times higher in the L-ω3 diet than the H-ω3 one. This was reflected in the fish after 12 weeks when again the ω6:ω3 fatty acid ratio showed the greatest difference among the fatty acid sums and ratios. This time the value was 40% higher in the L-ω3 fish than the H-ω3 ones. This ratio in diets and trout muscle together with individual ω6 and ω3 polyunsaturated fatty acid proportions is shown in Figure 2. Proportions of 18:2ω6 showed the greatest difference among the PUFA in the diets (Table 2) being 4 times higher in the L-ω3 diet than the H-ω3 one. This was again reflected in a more muted way in the muscle at the end of the feeding trial (Figure 2b).
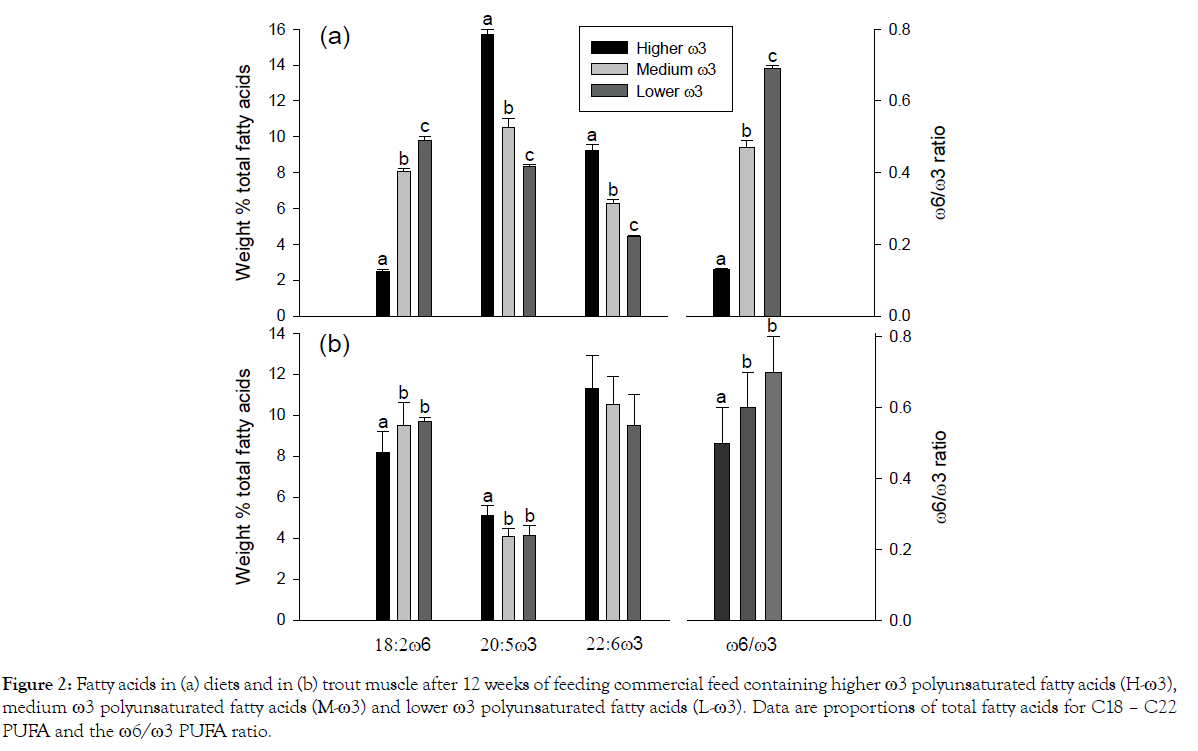
Figure 2: Fatty acids in (a) diets and in (b) trout muscle after 12 weeks of feeding commercial feed containing higher ω3 polyunsaturated fatty acids (H-ω3), medium ω3 polyunsaturated fatty acids (M-ω3) and lower ω3 polyunsaturated fatty acids (L-ω3). Data are proportions of total fatty acids for C18 – C22 PUFA and the ω6/ω3 PUFA ratio.
Significant changes in fatty acid percentages or ratios frequently occurred with drops in temperature (Table 4), but increase in temperature from 16.5°C to 18.0°C significantly decreased both terrestrial fatty acids and ω6:ω3 ratios in H-ω3 fed fish compared to those fed the L-ω3 diet (p ≤ 0.011). The tissue composition of marine origin fatty acids (EPA + DPA + DHA), Σω3, P:S, DHA, DPA and EPA increased significantly in H-ω3 fed fish with a corresponding decrease in fish fed L-ω3 diet (p ≤ 0.045).
Twelve weeks of feeding resulted in significant muscle fatty acid changes in fish fed all three diets (Table 4). The marine fatty acids, EPA:AA ratios, Ʃω3, EPA and 14:0 increased in H-ω3 fed fish with a corresponding decrease in L-ω3 fed fish (p ≤ 0.033). In contrast, terrestrial fatty acids, ω6:ω3 ratios and 18:1ω9 increased in L-ω3 fed fish with a corresponding decrease with the H-ω3 diet (p ≤ 0.030).
Significant changes in muscle fatty acid composition of M-ω3 fed fish occurred with increasing temperature to 18.0°C from the initial 13.5°C (Table 4). There was a corresponding decrease in terrestrial fatty acids, mainly 18:2ω6 (p ≤ 0.024). Subsequent drops in temperature to 13.5°C, decreased the total SFA composition by decreasing 14:0 and 16:0 in muscle tissue (p ≤ 0.018). The composition of 18:1ω9 increased with the decrease to 13.5°C (p = 0.027). Total PUFA in muscle tissue was not affected by diet at any temperature, but amounts of both 18:2ω6 and the ω6:ω3 ratios increased with the final decline to 13.5°C (p ≤ 0.03). Proportions of 16:0 and 18:1ω9 were affected (p ≤ 0.037) in L-ω3 fish on decreasing to 13.5°C (Table 4). 18:1ω9 increased with decreasing temperature while 16:0 decreased.
Overall 18:2ω6 and the sum of 18:2ω6 and 18:3ω3 consistently decreased with increasing temperature and then increased with decreasing temperature with all dietary treatments (Table 4). M-ω3 fed fish showed additional significant differences with temperature for 18:0 and 18:1ω9 (Figure 3) with the latter following the overall pattern for 18:2ω6 (Table 4) and 18:0 following the opposite trend. Correlation analysis confirmed the overall importance of saturated and monounsaturated fatty acids in response to temperature with 16:0 and ΣSFA showing strong positive responses among all diets (P=0.003-0.009) and 18:1ω9 and ΣMUFA showing negative ones (p=0.019-0.035).
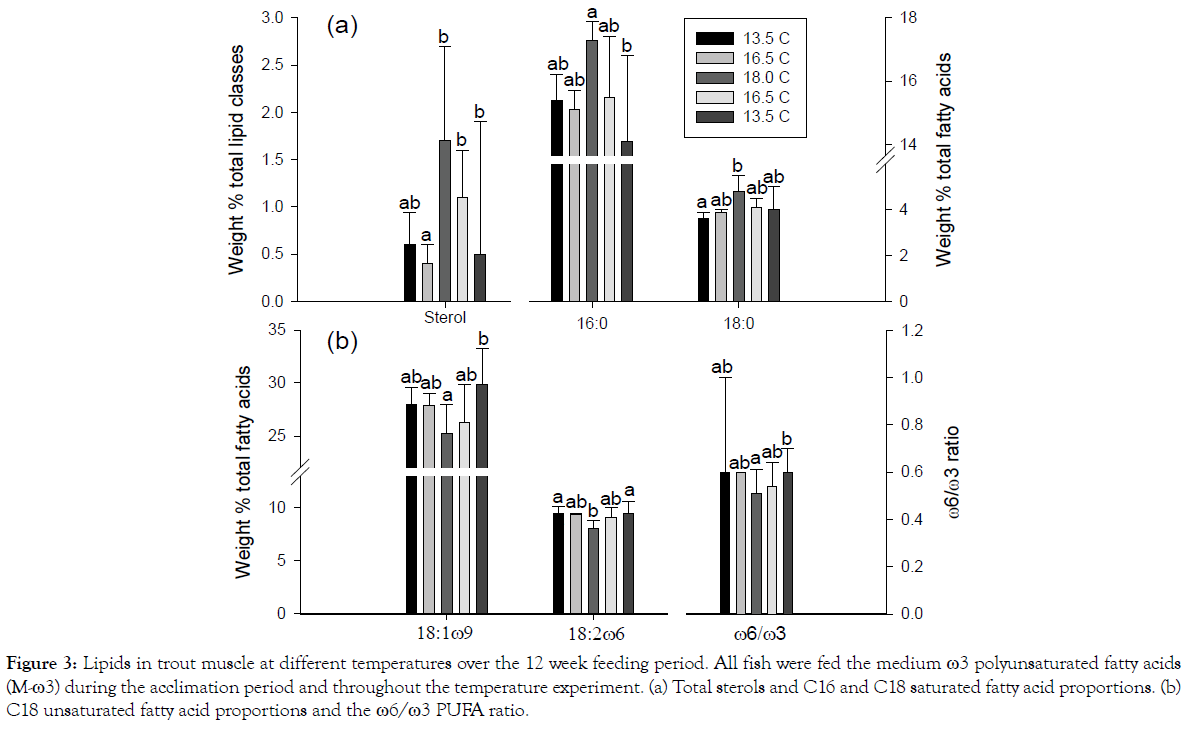
Figure 3: Lipids in trout muscle at different temperatures over the 12 week feeding period. All fish were fed the medium ω3 polyunsaturated fatty acids (M-ω3) during the acclimation period and throughout the temperature experiment. (a) Total sterols and C16 and C18 saturated fatty acid proportions. (b) C18 unsaturated fatty acid proportions and the ω6/ω3 PUFA ratio.
Figure 3 compares the effect of temperature on sterol and C18 fatty acid proportions in fish which were not subjected to a change in diet. The highest values for sterol and 18:0 occurred at the highest temperature which coincided with the lowest values for the C18 unsaturated fatty acids.
Discussion
Lipid class and fatty acid analysis indicated the qualitative difference between the 3 commercial diets used in this study. Total lipid was higher than the crude fat (min) % reported in the label information for the three feeds probably due to reporting minimum crude fat content in diets. The H-ω3 diet had half again, and nearly twice as much ω3 fatty acids over M-ω3 and L-ω3 diets, respectively, and the M-ω3 diet had a third more ω3 than L-ω3 diet. Marine origin oils were partially replaced in the L-ω3 diet resulting in higher MUFA and lower P:S ratios. VO completely lacks ω3 long-chain PUFA but is rich in ω6 and ω9 shorter chain fatty acids [3]. Animal fats are rich in SFA and MUFA. The composition of SFA would vary with species of origin. Beef tallow has approximately twice as much SFA as poultry fat. Animal fat might also contain certain amounts of C18 PUFA based on dietary history of the source animal. Trushenski and Lochmann [37] reported 27.5% SFA in poultry fat with 21% PUFA (primarily 18:2ω6) and 47.5% SFA in beef tallow with less than 4% PUFA. Full or partial replacement of fish oil in marine aquaculture could also lead to the risk of net deficiency in essential fatty acids (EFA). Therefore supplementation of aquafeeds with some fish meal is necessary to fulfill the EFA requirement in most cultured species.
The diet had no effect on growth or the food conversion efficiency of adult steelhead trout. Similar findings were reported in previous studies in Atlantic salmon, where growth was not affected by replacing a portion of fish oil with vegetable oil in diets containing 45 to 50% crude protein primarily from fish meal [10,38].
The muscle lipid composition in this study was affected both by change in growth temperature and diet. TAG was the predominant lipid class in muscle tissue with all three diets. Lipid class composition in fish muscle reflects dietary lipid intake [39,40], environmental temperature [26], lipid digestibility and metabolism [38,41]. Vegetable oil rich L-ω3 fish at 18.0°C had more stored neutral lipids and sterols compared to fish fed H-ω3 diets, contributing to the higher total lipid composition. Fish fed the L-ω3 diet had significantly more ST until the decrease to 13.5°C. Cholesterol is the most common ST in animal lipid [42] and is a major component of cell membranes, while some is stored together with neutral lipids in muscle tissue.
Replacing the base diet (M-ω3) with fish oil rich H-ω3 resulted in significant changes in the lipid composition of H-ω3 fed fish later in the experiment, but total PUFA was not affected by feed or temperature. L-ω3 and M-ω3 fed fish accumulated 18:1ω9 in muscle with decreasing temperature and M-ω3 fed fish accumulated both 18:1ω9 and 18:2ω6.
Studies examining simultaneous influences of diet and temperature on trout are few [43]. Using two diets and two temperatures Mellery et al. [27] examined the effect on rainbow trout fatty acid concentrations (mg/g) and it can be calculated that both the MUFA proportions (%) and the ω6/ω3 ratio decrease with increasing temperature independent of diet, as seen here.
Most studies have been conducted by acclimating salmon to two distinct temperatures with a primary focus on determining fatty acid changes in PLs [25,44,45] Fish studies with simultaneous manipulation of temperature and diet have given inconsistent results for various reasons such as species differences, types of oils and fat used in feeds and types of lipid classes and fatty acid investigated [43,45-47]. Nonetheless the fatty acid data in Figure 3 are largely consistent with those of Jobling and Bendiksen [25] who examined the interaction dietary lipids and temperature on neutral and polar lipids in Atlantic salmon. They saw similar temperature responses in parr, especially for 18:1ω9 and 18:2ω6 in those fed vegetable oil. Strong relationships with temperature for these two fatty acids have also been found in juvenile trout [48,49].
The significantly higher levels of 16:0 and 18:0, and the significantly lower levels of 18:1ω9, 18:2ω6, and ω6/ω3 ratio with increasing temperature (Figure 3) seem to be a more general response in fish. Cobia fillets followed the almost exact same pattern independent of diet [28], and by far the lowest values of 18:2ω6 and ω6/ω3 ratio occurred at the highest temperature in golden mahseer [30].
The significant diet-independent correlations of ΣSAT (r=0.69), 16:0 (r=0.74) and 18:1ω9 (r=-0.56) with temperature are consistent with 14C label experiments in livers from thermally acclimated fish. In the phospholipid and triacylglycerol fatty acids of rainbow trout, the label was incorporated primarily into 18:1ω9 and 16:0 at two different temperatures but overall there was significantly more incorporated into saturated fatty acids in response to warming [50]. In carp, increase in temperature again gives rise to de novo synthesis of saturated fatty acids, especially palmitic acid, 16:0 [51]. Also in carp, a 7°C cooling increased Δ9-desaturase activity within a day [52]. This enzyme introduces the first double bond into a fatty acid at the central C9-C10 position which in the C18 saturate, stearic acid (18:0), leads to the ω9 monounsaturate, oleic acid (18:1ω9).
While 85-94% of the lipids in trout muscle are in triacylglycerols (Table 2) the fatty acid changes with temperature have implications for maintenance of membrane physical characteristics. Substitution of a Δ9-unsaturated fatty acid (e.g. 18:1ω9) for a saturated one (e.g. 16:0) in membrane phospholipids would have a maximal disordering effect on physical properties [22] rendering the membranes less packed and more fluid on cooling. Similarly, the increase in sterol at 18°C (Figure 3) could help stabilize the membranes at high temperature [22,29].
Studies conducted to date primarily focus on determining the effects of diet and temperature on the polar lipid fraction due to its significance in membranes [21-23,53,54]. Since similar changes were also seen in muscle in this study with over 80% of lipids being stored as neutral TAG it is likely that this is more than just a membrane response.
Both M-ω3 and L-ω3 fed fish had a similar response to temperature by manipulating only shorter chain MUFA or PUFA (18:1ω9 and 18:2ω6). Temperature had no significant influence on marine lipids, ω3 or P:S ratio in M-ω3 or L-ω3 fed fish. Stubhaug et al. [2] showed salmon could selectively store long chain PUFA with low dietary intake. They suggested that fish primarily locate EPA and DHA in membranes with low dietary intake while switching the fatty acid substrate used for β-oxidation. This further stresses the fact that cold water fish use surplus fatty acids for energy production while storing the essential fatty acids that are limited [2,10], hence highlighting the lack of response in long-chain PUFA.
Conclusion
This is the first study to evaluate the effect of fluctuating temperature on muscle lipid and fatty acid composition in steelhead trout under commercial feeding conditions. Research conducted to date has primarily focused on two temperatures using strict experimental diets or comparing such diets with a commercially available diet. Here farm-sourced adult trout were fed commercial diets over a natural temperature cycle. Although substituting FO with plant or animal origin lipids did not affect total PUFA levels in muscle tissue, ω6:ω3 ratios changed considerably, with implications for seafood quality. Fish fed all diets responded to temperature perturbation by changing total lipid concentrations and C18 fatty acid proportions; however, 16:0 showed the strongest correlation with temperature. This study shows that commercially available diets with similar label information could yield fillets with very different ω6:ω3 fatty acid ratios and that growth temperature directly influences fatty acid quantity and composition in trout muscle tissue. Comparison with the literature indicates increased 16:0 and 18:0 and lower 18:1ω9, 18:2ω6, and ω6/ω3 ratio is a general response in fish to higher temperatures.
Acknowledgment
We would like to thank the Dr. Joe Brown Aquatic Research Building staff at the Ocean Sciences Centre for providing infrastructure for rearing fish, and Jeanette Wells for technical assistance with lipid analysis. This research was funded by the Department of Fisheries and Oceans, Canada and NSERC.
REFERENCES
- Ng WK, Tocher DR, Bell JG. The use of palm oil in aquaculture feeds for salmonid species. Eur J Lipid Sci Technol. 2007; 109(4):394-9.
- Stubhaug I, Lie Ø, Torstensen BE. Fatty acid productive value and β‐ oxidation capacity in Atlantic salmon (Salmo salar L.) fed on different lipid sources along the whole growth period. Aquac Nutr. 2007; 13(2):145-55.
- Turchini GM, Torstensen BE, Ng WK. Fish oil replacement in finfish nutrition. Rev Aquac. 2009; 1(1):10-57.
- Turchini GM, Mentasti T, Frøyland L, Orban E, Caprino F, Moretti VM, et al. Effects of alternative dietary lipid sources on performance, tissue chemical composition, mitochondrial fatty acid oxidation capabilities and sensory characteristics in brown trout (Salmo trutta L.). Aquac. 2003; 225(1-4):251-67.
- Bureau D, Gibson J. Animal fats as aquaculture feed ingredients: nutritive value, product quality and safety. Aquafeed Int. 2004; 7:32-7.
- Bell JG, Henderson RJ, Tocher DR, Sargent JR. Replacement of dietary fish oil with increasing levels of linseed oil: modification of flesh fatty acid compositions in Atlantic salmon (Salmo salar) using a fish oil finishing diet. Lipids. 2004; 39(3):223-32.
- Torstensen BE, Frøyland L, Lie Ø. Replacing dietary fish oil with increasing levels of rapeseed oil and olive oil–effects on Atlantic salmon (Salmo salar L.) tissue and lipoprotein lipid composition and lipogenic enzyme activities. Aquac Nutr. 2004; 10(3):175-92.
- Torstensen BE, Bell JG, Rosenlund G, Henderson RJ, Graff IE, Tocher DR, Lie Ø, et al. Tailoring of Atlantic salmon (Salmo salar L.) flesh lipid composition and sensory quality by replacing fish oil with a vegetable oil blend. J Agric Food Chem. 2005; 53(26):10166-78.
- Nanton DA, Vegusdal A, Rørå AM, Ruyter B, Baeverfjord G, Torstensen BE. Muscle lipid storage pattern, composition, and adipocyte distribution in different parts of Atlantic salmon (Salmo salar) fed fish oil and vegetable oil. Aquac. 2007; 265(1-4):230-43.
- Bell JG, McGhee F, Campbell PJ, Sargent JR. Rapeseed oil as an alternative to marine fish oil in diets of post-smolt Atlantic salmon (Salmo salar): changes in flesh fatty acid composition and effectiveness of subsequent fish oil “wash out”. Aquac. 2003; 218(1-4):515-28.
- Regost C, Jakobsen JV, Rørå AM. Flesh quality of raw and smoked fillets of Atlantic salmon as influenced by dietary oil sources and frozen storage. Food Res Int. 2004; 37(3):259-71.
- Regost C, Arzel J, Cardinal M, Rosenlund G, Kaushik SJ. Total replacement of fish oil by soybean or linseed oil with a return to fish oil in Turbot (Psetta maxima): 2. Flesh quality properties. Aquac. 2003; 220(1-4):737-47.
- Mørkøre T. Relevance of dietary oil source for contraction and quality of pre-rigor filleted Atlantic cod, Gadus morhua. Aquac. 2006; 251(1):56-65.
- Vegusdal A, Gjøen T, Berge RK, Thomassen MS, Ruyter B. Effect of 18∶ 1n-9, 20∶ 5n-3, and 22∶ 6n-3 on lipid accumulation and secretion by atlantic salmon hepatocytes. Lipids. 2005; 40(5):477-86.
- Thompson KD, Tatner MF, Henderson RJ. Effects of dietary (n‐3) and (n‐6) polyunsaturated fatty acid ratio on the immune response of Atlantic salmon, Salmo salar L. Aquac Nutr. 1996; 2(1):21-31.
- Bransden MP, Carter CG, Nichols PD. Replacement of fish oil with sunflower oil in feeds for Atlantic salmon (Salmo salar L.): effect on growth performance, tissue fatty acid composition and disease resistance. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 2003; 135(4):611-25.
- Pickova J, Mørkøre T. Alternate oils in fish feeds. Eur J Lipid Sci Technol. 2007; 109(3):256-63.
- Wijendran V, Hayes KC. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu Rev Nutr. 2004; 24:597-615.
- Myers JL, Allen JC. Nutrition and inflammation: insights on dietary pattern, obesity, and asthma. Am J Lifestyle Med. 2012; 6(1):14-7.
- Loef M, Walach H. The omega-6/omega-3 ratio and dementia or cognitive decline: a systematic review on human studies and biological evidence. J Nutr Gerontol Geriatr. 2013; 32(1):1-23
- Farkas T, Fodor E, Kitajka K, Halver JE. Response of fish membranes to environmental temperature. Aquac Res. 2001; 32(8):645-55.
- Hazel JR, Williams EE. The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Res. 1990; 29(3):167-227.
- Fodor E, Jones RH, Buda C, Kitajka K, Dey I, Farkas T. Molecular architecture and biophysical properties of phospholipids during thermal adaptation in fish: an experimental and model study. Lipids. 1995; 30(12):1119-26.
- Hazel JR. Influence of thermal acclimation on membrane lipid composition of rainbow trout liver. Am J Physiol. 1979; 236(1):R91- 101.
- Jobling M, Bendiksen EÅ. Dietary lipids and temperature interact to influence tissue fatty acid compositions of Atlantic salmon, Salmo salar L., parr. Aquac Res. 2003; 34(15):1423-41.
- Skalli A, Robin JH, Le Bayon N, Le Delliou H, Person-Le Ruyet J. Impact of essential fatty acid deficiency and temperature on tissues' fatty acid composition of European sea bass (Dicentrarchus labrax). Aquac. 2006; 255(1-4):223-32.
- Mellery J, Geay F, Tocher DR, Kestemont P, Debier C, Rollin X, et al. Temperature increase negatively affects the fatty acid bioconversion capacity of rainbow trout (Oncorhynchus mykiss) fed a linseed oil-based diet. PloS one. 2016; 11(10):e0164478.
- Araújo BC, Honji RM, Rombenso AN, De Souza GB, De Mello PH, Hilsdorf AW, et al. Influences of different arachidonic acid levels and temperature on the growth performance, fatty acid profile, liver morphology and expression of lipid genes in cobia (Rachycentron canadum) juveniles. Aquac. 2019; 511:734245.
- Wijekoon MP, Parrish CC, Gallardi D, Nag K, Mansour A. Diet and temperature affect liver lipids and membrane properties in steelhead trout (Oncorhynchus mykiss). Aquaculture Nutrition. 2021; 27(3):734-46.
- Akhtar MS, Pal AK, Sahu NP, Ciji A, Mahanta PC. Higher acclimation temperature modulates the composition of muscle fatty acid of Tor putitora juveniles. Weather Clim Extr. 2014; 4:19-21.
- Hoegh-Guldberg O, Jacob D, Taylor M, Bolaños TG, Bindi M, Brown S, et al. The human imperative of stabilizing global climate change at 1.5 C. Science. 2019; 365(6459).
- https://climefish.eu/climate-change-and-impacts-on-aquaculture/
- Parrish CC. Determination of total lipid, lipid classes, and fatty acids in aquatic samples. Lipids in Freshwater ecosystems. Springer. 1999; pp: 4-20.
- Folch J, Lees M, Stanley GS. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226(1):497-509.
- Parrish CC. Separation of aquatic lipid classes by Chromarod thin- layer chromatography with measurement by latroscan flame ionization detection. Can J Fish Aquat Sci. 1987; 44 (4): 722-31.
- Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J Lipid Res. 1964; 5(4):600-8.
- Trushenski JT, Lochmann RT. Potential, implications and solutions regarding the use of rendered animal fats in aquafeeds. Am J Anim Vet Sci. 2009; 4(4):108-28.
- Torstensen BE, Lie Ø, Frøyland L. Lipid metabolism and tissue composition in Atlantic salmon (Salmo salar L.)—effects of capelin oil, palm oil, and oleic acid-enriched sunflower oil as dietary lipid sources. Lipids. 2000; 35(6):653-64.
- Higgs DA, Dong FM. Lipids and fatty acids. In: Encyclopaedia of Aquaculture. Stickney RR (ed), John Wiley & Sons, New York. 2000: pp: 476–496.
- Sargent JR, Tocher DR, Bell JG, Halver JE, Hardy RW. Fish nutrition. Lipids. 2002; pp:182-257.
- Bell JG, Henderson RJ, Tocher DR, McGhee F, Dick JR, Porter A, et al. Substituting fish oil with crude palm oil in the diet of Atlantic salmon (Salmo salar) affects muscle fatty acid composition and hepatic fatty acid metabolism. J Nutr. 2002; 132(2):222-30.
- Tocher DR. Metabolism and functions of lipids and fatty acids in teleost fish. Rev Fish Sci. 2003; 11(2):107-84.
- Labbé C, Maisse G, Müller K, Zachowski A, Kaushik S, Loir M. Thermal acclimation and dietary lipids alter the composition, but not fluidity, of trout sperm plasma membrane. Lipids. 1995; 30(1):23-33.
- Skuladottir GV, Schiöth HB, Gudmundsdottir E, Richards B, Gardarsson F, Jonsson L. Fatty acid composition of muscle, heart and liver lipids in Atlantic salmon, Salmo salar, at extremely low environmental temperature. Aquac. 1990; 84(1):71-80.
- Grisdale-Helland B, Ruyter B, Rosenlund G, Obach A, Helland SJ, Sandberg MG, et al. Influence of high contents of dietary soybean oil on growth, feed utilization, tissue fatty acid composition, heart histology and standard oxygen consumption of Atlantic salmon (Salmo salar) raised at two temperatures. Aquac. 2002; 207(3-4):311-29.
- Craig SR, Neill WH, Gatlin DM. Effects of dietary lipid and environmental salinity on growth, body composition, and cold tolerance of juvenile red drum (Sciaenops ocellatus). Fish Physiol Biochem. 1995; 14(1):49-61.
- Fracalossi DM, Lovell RT. Growth and liver polar fatty acid composition of year-1 channel catfish fed various lipid sources at two water temperatures. Progressive Fish-Culturist. 1995; 57(2):107-13.
- Parrish C, Hixson S, Wijekoon M, Anderson D. Plant lipid, protein use in cod, salmonid diets.
- Parrish CC. Essential fatty acids in aquaculture. Int Aquafeed Magazine. 2018; pp:16-17.
- Hazel JR, Prosser CL. Incorporation of 1-14C-acetate into fatty acids and sterols by isolated hepatocytes of thermally acclimated rainbow trout (Salmo gairdneri). J Comp Physiol. 1979; 134(4):321-9.
- Farkas T, Csenger I. Biosynthesis of fatty acids by the carp, Cyprinus carpio L., in relation to environmental temperature. Lipids. 1976; 11(5):401-7.
- Trueman RJ, Tiku PE, Caddick MX, Cossins AR. Thermal thresholds of lipid restructuring and delta (9)-desaturase expression in the liver of carp (Cyprinus carpio L.). J Exp Biol. 2000; 203(3):641-50.
- Logue JA, De Vries AL, Fodor EL, Cossins AR. Lipid compositional correlates of temperature-adaptive interspecific differences in membrane physical structure. J Exp Biol. 2000; 203(14):2105-15.
- Hochachka PW, Somero GN. Biochemical Adaptation: Oxford university press. 2002.
Citation: Wijekoon M, Parrish CC, Mansour A (2021) Effect of Growth Temperature on Muscle Lipid Class and Fatty Acid Composition in Adult Steelhead Trout (Oncorhynchus mykiss) Fed Commercial Diets with Different ω6 to ω3 Fatty Acid Ratios. J Aquac Res Development. 12: 643.
Copyright: © 2021 Wijekoon M, et al. This is an open access article distributed under the term of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.