Indexed In
- Open J Gate
- JournalTOCs
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2019) Volume 7, Issue 2
Effect of Graphite and Copper Nano-Particles on Free Volume Properties of PVC/NBR Blends Studied by Pal Spectroscopy
Ehsan Gomaa* and Emad Hassan AliReceived: 28-Nov-2019 Published: 31-Dec-2019
Abstract
Positron Annihilation Lifetime (PAL) spectroscopy has been used to investigate the effect of different concentrations of graphite and copper nano-particles in polyvinylchloride (PVC)/ butadiene-acrylonitrile copolymer (NBR) blends on free volume properties of PVC/NBR nano composites. However, the miscibility of PVC/NBR blends was investigated. The free-volume properties showed a negative deviation from linear additive relationship indicating miscibility of two blends. In additions, this research to confirm the results of the previous study on the same samples through the correlation between positron annihilation parameters and some of the properties studied.
Keywords
Positron annihilation lifetime; Free-volume holes; Blend; Butadiene-acrylonitrile copolymer (NBR); Polyvinyl chloride (PVC); Graphite; Copper; Nano-particles
Introduction
The addition of polyvinyl chloride (PVC) to butadiene-acrylonitrile copolymer (NBR) make a kind of rubber-plastic mixture which may have more desirable properties and reduce the cost. In the NBR/PVC blend, an increasing PVC content would accompany with an increase of the mechanical and thermal properties. The main advantage of NBR/PVC blends over NBR is that they have excellent resistance to ozone.
Graphite, which is naturally abundant, has been widely used as conducting filler in preparing conducting polymer composites [1-3]. Conventional graphite fillers are usually micro-diameter powders. In order to get a satisfactory conductivity, using conventional graphite fillers, and composite loadings are usually as high as 15-20 wt% or even higher. This often results in a material with poor mechanical properties and high density. Like many other nanocomposites, a polymer nanocomposite made from graphite nanopowder or nanosheets may have promising properties, especially good electrical conductivity.
When positrons enter a condensed medium like a polymer, they thermalize very rapidly by losing their energy by collisions with the molecules. Thermalized positrons annihilate with electrons of the medium. The positron can also form a bound state with an electron, called positronium (Ps), which can exist in two spin states. The singlet para-positronium state (p-Ps) with antiparallel spins has a self-annihilation lifetime of 0.125 ns and it decays by the emission of two photons. The triplet ortho-positronium state (o-Ps) with parallel spins has a considerably longer lifetime and it annihilates in free space into three photons with a lifetime of 140 ns in vacuum. In polymers, this o-Ps annihilates predominantly via a fast channel called pick-off annihilation (with an outside electron of the medium) by two-photon emission and its lifetime gets reduced to 1-5 ns. The o-Ps pick-off lifetime depends on the overlap of the Ps wave-function with the wave-function of the electron in the freevolume cavity. The larger the cavity size, the smaller is the overlap and hence the longer is the lifetime [4]. Thus, the formation of o-Ps and its yield in polymers is determined by positron lifetime measurement, attributing the long-lived component to o-Ps decay [5].
Experimental Procedure
Materials
PVC resin is a white powder made by suspension polymerization with K value of 67. It was supplied by El-Amerria Company (Alexandria, Egypt). Acrylonitrile butadiene copolymer (NBR) from Bayer AG, Germany with 32% acrylonitrile content and specific gravity 1.17 ± 0.005 at room temperature was used. Tetrahydrofuran (THF) and chloroform were reagent grade from Merck, Darmstadt, Germany. The fine grade of copper with average particle size varies between 60-75 nm was obtained from BDH chemicals Ltd, Poole, England.
Nano graphite powder produced by chemical disintegration of graphite with average particle size 40 nm supplied by Nanjing Emperor Nano Material Co., Ltd, China.
Preparation of NBR/PVC blends by the solution-casting The required polymer solutions were prepared by the dissolution of 5 g of the PVC in 100 ml of THF and 1.2 g of NBR in 100 ml chloroform separately. Polymer blend films were prepared through the casting of mixed solutions of different ratios (0/100, 10/90, 20/80, 30/70, 40/60, and 50/50) of NBR to PVC on a glass plate with slow drying at room temperature. Care was taken to control the uniform film thickness for all compositions. The film thickness ranging from 0.35 to 0.45 cm was controlled by using the same solution concentration of the polymers under investigation.
Preparation of NBR/PVC nanocomposites
PVC/NBR nanocomposites were prepared by the addition of 1, 3 and 5 wt% of copper or graphite to the previous prepared PVC/ NBR blends in the same manner as aforementioned.
Positron annihilation lifetime measurement
Positron lifetime measurements were carried out at room temperature using a 11 μ Ci22 Na source sealed between two kapton foils (thickness less than 1 mg/cm2) with a small active diameter of l-2 mm in sandwich geometry with the pellets and a standard fast-fast coincidence lifetime spectrometer. Two identical plastic scintillator detectors fitted with Hamamatsu photomultiplier tubes [H3378-50]NO. BA0828 with a prompt resolution of about 250 ps (full width at half-maximum, FWHM) was used in the present study. Lifetime spectra were recorded for each sample with about 5 × 106 counts accumulated under the peak. After source correction was determined using a properly defect free Silicon sample, the lifetime spectra were analyzed in three components, using the computer program LT 9.0 [6] which deduced finite and long-term distribution analysis.
The three lifetime components are: the para-positronium (p-Ps) component with lifetimes (τ1) and intensity (I1), free positron components with lifetime and intensity (τ2 & I2) and the orthopositronium (o-Ps) components (τ3 & I3) for the which is due to pick-off annihilation in free volumes. The mean free-volume radius (R) of holes can be calculated by using the following semi empirical equation [4,5].
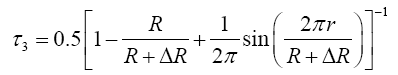
Where ΔR=1.66 Å is the fitted empirical constant [5].
By fitting the above equation with the measured τ3, values of R
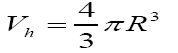
and can be calculated (where the free-volume holes are assumed to be spherical). The relative intensity of the longest component, I3, is generally correlated to the fractions of holes which can be considered as trapping centres for Ps.
A semi-empirical relation may be used to determine the relative fraction of the free-volume holes (F%) in polymers [4] as follows: F=Vh I3
The o-Ps annihilation lifetime distributions obtained from LT 9.0 analysis by using a spherical approximation of free-volume holes, the free- volume probability density fraction as [7].
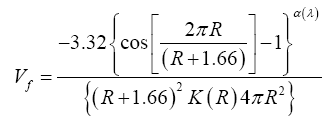
Vf (pdf) is the free-volume hole probability density function. K (R) (K (R)=1.0+8.0 R, where 8.0 is taken from the ratio between the positron trapping rate and the hole radius of voids) is a correlation factor for the Ps trapping probability as a function of hole radius R from the positron-void size relationship [8]. The fraction of hole volume between Vf and dVf is Vf (pdf) dVf. Detailed descriptions in this regard can be found elsewhere [7].
Results and Discussion
Free-volume parameters in pure polymers
The free-volume hole parameters in pure PVC and pure NBR are listed in Table 1. The results showed that PVC has a smaller size and low fraction of free-volume holes than the corresponding freevolume values of NBR. This is due to the chemical structure of each polymer and also the degree of branching of the NBR. The differences in sizes of the free-volume holes between two polymers can be seen more clearly in the free-volume hole size distributions where the results of the free volume probability density function Vf(pdf) are shown in Figure 1 for two pure polymers, PVC and NBR.
| Polymer | τ3 ns | I3 % | Vf (Å)3 | F% |
|---|---|---|---|---|
| PVC | 2.1905 ± 0.007 | 6.036 ± 0.023 | 115.72 ± 0.40 | 1.1980 ± 0.011 |
| NBR | 2.323 ± 0.026 | 18.20 ± 0.53 | 127.83 ± 0.17 | 4.1882 ± 0.013 |
Table 1: Free-volume parameters in pure PVC and pure NBR.
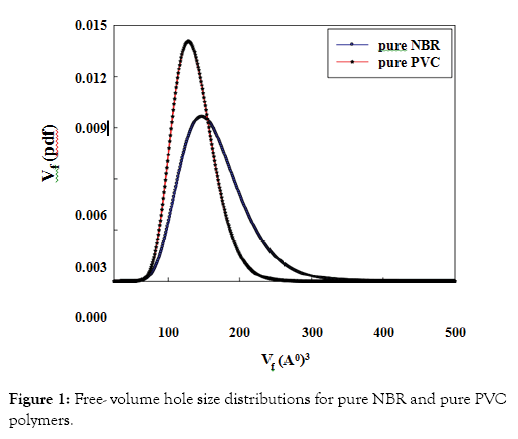
Figure 1: Free- volume hole size distributions for pure NBR and pure PVC polymers.
Free-volume parameters in PVC/NBR blends
The miscibility of PVC/NBR blend: Previous works [9,10] used the free-volume parameters as a method to exam the miscibility or immiscibility of polymer blends. Therefore, if the free-volume parameters of the blends show a positive deviation from the well-known linear additively rule, this means that the blend is immiscible [11], whereas the deviation is negative, it means that the blend is miscible [10,12]. The miscible polymer blend acquires homogeneous nature and it is a single-phase system represents compact packing of the polymeric segments due to change in chain conformation [9]. Figures 2a and 2b is a plot of average size, τ3, of free-volume hole and its fraction, I3, as a function of wt% of NBR in the PVC/NBR blend. The dotted line represents the linear additive relationship. The observed change in the τ3 and I3 shows negative deviation from additive rule which can be used to conclude that PVC/NBR blend is miscible. Since, the free-volume parameters (τ3 and I3) deviate negatively from the additively rule suggests improved interaction between the blend constituents. This happens due to the carboxyl group (proton acceptor) in NBR capable of specific interaction with the alpha hydrogen of PVC which leads to miscibility of the two polymers. On the other hand, we notice that as the NBR concentration is increased in the blend, the size (τ3) of free-volume holes decreases and it is well below the expected values from the additively rule, indicating comparatively close packing of the chain segments.
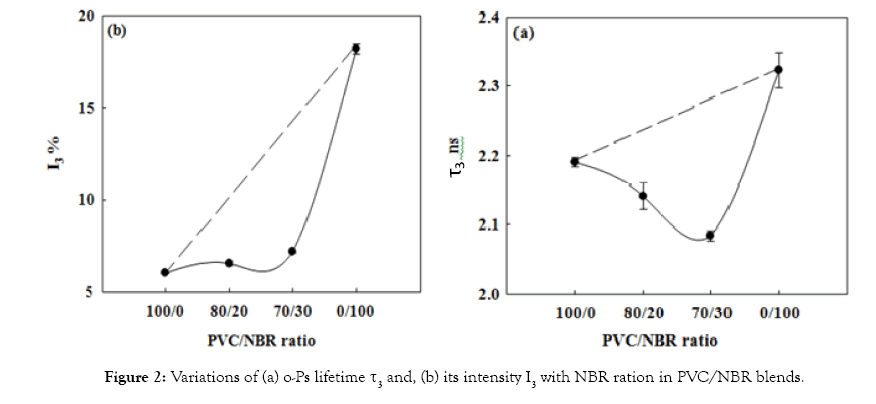
Figure 2: Variations of (a) o-Ps lifetime τ3 and, (b) its intensity I3 with NBR ration in PVC/NBR blends.
Lifetime distribution of PVC/NBR blend: The miscibility between two polymers can be seen more clearly in the free-volume hole size distributions. Figure 3 represents the free-volume hole size distributions of PVC/NBR blends. In the figure, it is observed that the blend is expected to exhibit a narrower distribution at 20% and 30% NBR and the distributions are shifted to the left indicating the compatibility and homogeneity of these compositions. The observed trends are consistent with the results obtained by finite analysis (Figure 2a).
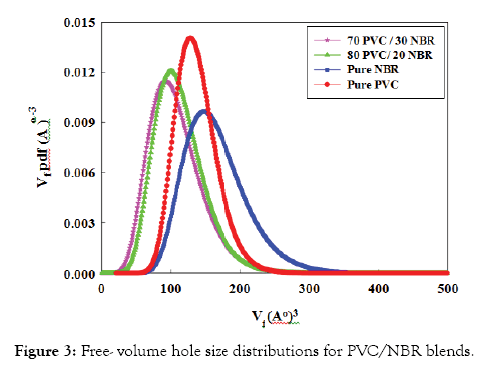
Figure 3: Free- volume hole size distributions for PVC/NBR blends.
Free-volume properties in PVC/NBR nanocomposites
Variation of positron annihilation lifetime parameters with the filler content: The dependence of positron annihilation lifetime parameters (τ2, I2, τ3 and I3) of PVC/NBR blends on the nano-grapite and nano-copper contents is shown in Figures 4 and 5. From these figures, three stages can be distinguished of the nanocomposites. The first stage from 100 PVC/0 NBR to 80 PVC/20 NBR while the second stage lies between 80 PVC/20 NBR to 60 PVC/40 NBR and the third stage is between 60 PVC/40 NBR and 50 PVC/50 NBR. Some general observations can be deduced from the results;
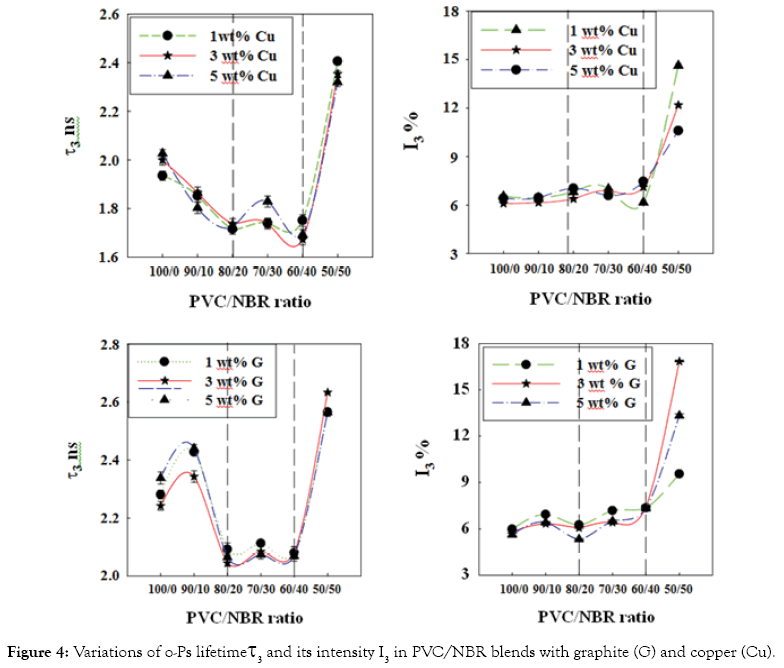
Figure 4: Variations of o-Ps lifetime τ3 and its intensity I3 in PVC/NBR blends with graphite (G) and copper (Cu).
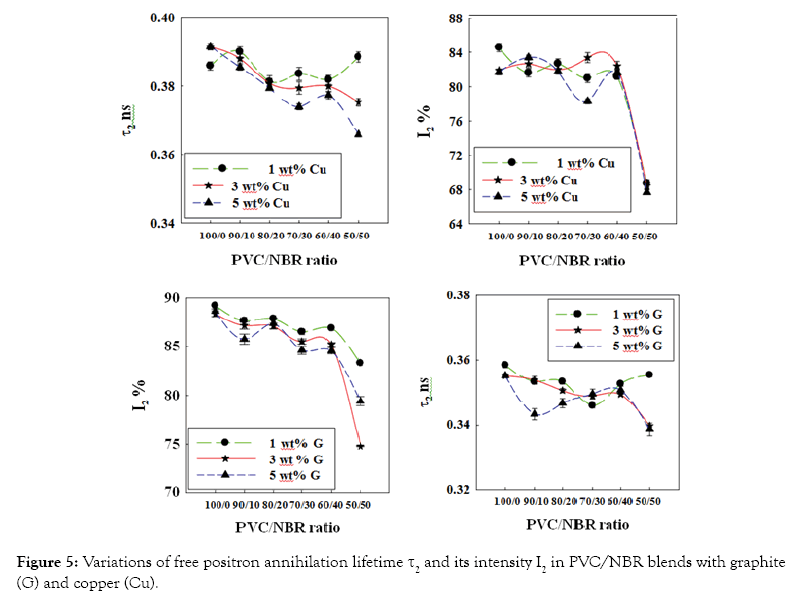
Figure 5: Variations of free positron annihilation lifetime τ2 and its intensity I2 in PVC/NBR blends with graphite (G) and copper (Cu).
I. The behaviour of the positron annihilation parameters are almost the same as a function of copper (Cu) and graphite (G) content through the three stages.
II. Highest value of free-volume parameters (τ3 and I3) and lowest value of the free positron annihilation parameters (τ2 and I2) at 50/50 PVC/NBR nanocomposites.
III. Free-volume parameters for the graphite (G) filled composites have higher values than those of copper (Cu) filled composites. Regarding to the third observation, the particle size of the filler and its surface area can change highly the physical properties of the composite [13]. Since graphite has smaller particle size (40 nm) and larger surface area than those of Cu (60 nm), so the probability of filling the free- volume holes is expected to be smaller than that in Cu composites, which results in larger values of free-volume hole parameters.
During the first stage, the behaviour of PVC/NBR/Cu nanocomposites showed continuous decrease in free-volume hole size (τ3) while an increase follows by a sharp falling in case of PVC/ NBR/G between 90PVC/10NBR/G and 80PVC/20NBR/G, indicating that there is a change in the microstructure of the composites. To interpret this observation, it should be mentioned that adding fillers, Cu or G, to blend matrix inhibits the o-Ps formation due to filling up some of the free volume holes in blend matrix and as a result the size of these holes decrease [14,15]. Furthermore, the strong interaction between filler and PVC/NBR blend and the obstacles effect caused by the filler can effectively restrain the motion of polymer chains. On the other hand, layers structure of graphite is the reason for the behaviour of PVC/ NBR/G nanocomposites. The addition of the polymer chains in graphite cause separation of the layers, one another and the layers are dispersed within the polymer matrix, which increase inter layer spacing then an exfoliated structure is formed and this is confirmed by the first increase of free-volume hole parameters. Besides, the polar groups on graphite surface e.g. -OH and -COOH, might interact physically and chemically strong interfacial interactions with α-hydrogen of PVC [15], which reduced the mobility of the polymer leading to a sharp decrease in the size of free-volume holes as shown in (Figure 4).
In the second region, (80 PVC/20NBR to 60 PVC/40 NBR) the microstructure of the nanocomposite is unchanged in its appearance, where the free positron annihilation parameters are constant. This might be rearrangement of structure will occurs in a limit extent. However, in case of PVC/NBR/G nanocomposites may be the intercalated structure formed when the polymer is inserted in inters layer spacing of graphite which decreases the electrostatic force between the layers, and increases the spacing to allow the polymer enters between layers. Therefore, the graphite layers are still in array periodically called ordered intercalated nanocomposite which leads to formation of free-volume holes with constant sizes as shown in Figure 4.
On the other hand, during the two stages, no variation is observed in the fractions of free-volume holes (I3) and the fraction of the defects in crystalline amorphous region (I2) while a little decrease is observed in τ2 (Figures 4 and 5). In such case the positron has a higher probability to annihilate in the filler than to form o-Ps.
In third region, (60 PVC/40 NBR to 50 PVC/50 NBR) an abrupt increase in τ3 and I3 while a decreasing in τ2 and I2 is observed. This can be interpreted as follows: increasing of NBR content in nanocomposites decrease the stiffness of the blend which leads to increasing of the size and the fractions of free-volume holes. This also might be attributed to formation of clusters [15] of graphite and copper where NBR content increased in the blend. On the other hand, the present of filler and NBR increasing the polarity in nanocomposites which causes a decrease in annihilation of free positrons as observed as a sharp decrease in τ2 and I2.
To confirm the previous results [15] of mechanical and electrical properties on the same samples a correlation established between positron annihilation parameters and some of these properties. Ward et al. [15] concluded that the tensile strength decreases while elongation at break increases with increasing as the filler loading increases in all composite samples. In addition to the values of conductivity for the nanocomposite of 50 PVC/50 NBR filled with 5% copper belongs to materials characterized as semiconductors.
Figure 6 shows correlation between free-volume parameters (R and F%) with tensile strength and elongation at break as well as the intermediate lifetime components τ2 and I2) and electrical conductivity (σ). Figure 6 shows a negative correlation between free-volume parameters (R and F%) and tensile strength while a positive correlation is observed with elongation at break.
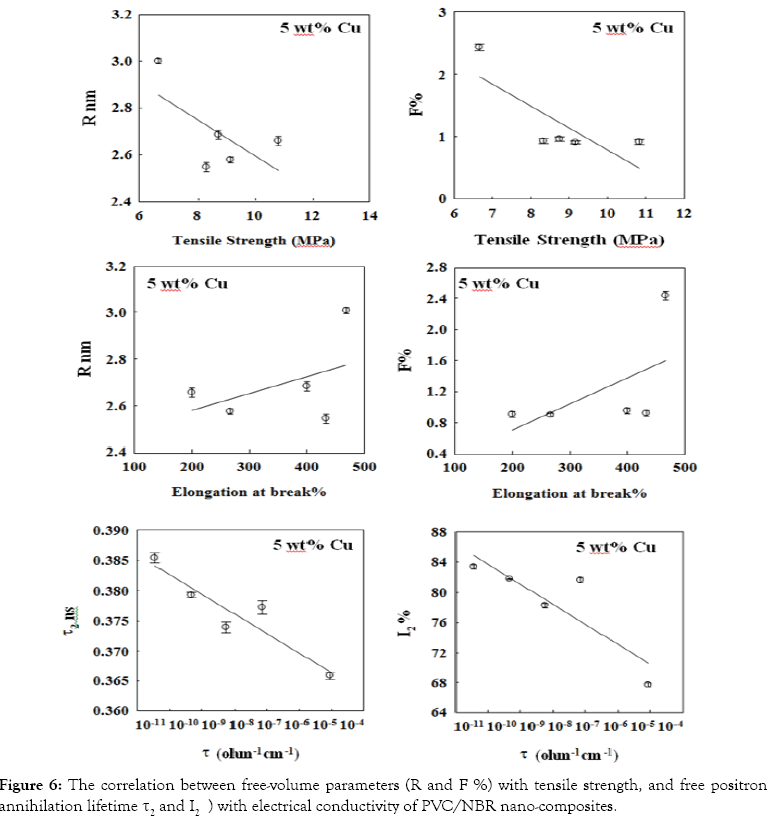
Figure 6: Legend
The increase of tensile strength at low concentration of filler is due to the good filler-blend interaction as a result of better filler dispersion in the polymer matrix. At high filler concentration, the interaction between filler and the blend matrix is hindered, resulting in low tensile strength and free volume hole parameters.
The addition of filler increases the electrical conductivity of nancomposite to semiconductor materials. However a negative correlation between free positron annihilation components (I2 and τ2) and σ was observed (Figure 6). This negative correlation is due to the polarity of the filler being higher than that of the blend itself which leads to an increase in electrical conductivity and an inhibition of free positrons annihilation.
Conclusions
The following conclusions can be drawn from the above discussion.
• PAL results showed that pure PVC has a smaller size and low fraction of free-volume holes than the corresponding free-volume values of pure NBR.
• The observed change in the τ3 and I3 shows negative deviation from additive rule which indicates that PVC/ NBR blend is miscible.
• The behaviour of the positron annihilation parameters are almost the same as a function of copper (Cu) and graphite (G) content.
• The behaviour of PVC/NBR/Cu nanocomposites showed continuous decrease in free-volume fraction (τ3) while an increase follows by a sharp falling in case of PVC/NBR/G between 90PVC/10NBR/G and 80PVC/20NBR/G, indicating that there is a change in the microstructure of the composites.
• Due to smaller particle size and larger surface area of G than those of Cu, the probability of filling the free-volume holes is smaller in G than that in Cu composites.
• The highest value of free-volume parameters (τ3 and I3) and lowest value of the free positron annihilation parameters (τ2 and I2) is observed at 50/50 PVC/NBR nanocomposites.
• The correlation between the mechanical, electrical properties and positron annihilation lifetime parameters confirmed the previous results by Ward et al. on the same samples.
Acknowledgement
The authors are indebted to S. Mansour and J. Assad (Polymers and Pigments Department, National Research Centre, Cairo, Egypt) for the samples supply. The authors are also, truly indebted to Professor Reinhard Krause-Rehberg for providing the positron experiment in the Martin-Luther-University Halle-Wittenberg, Germany.
REFERENCES
- Saunders DS, Galea SC, Deirmendjian GK. The development of fatigue damage around fastener holes in thick graphite/epoxy composite laminates. Composites. 1993;24:309-321.
- Chen GH, Wu DJ, Weng WG, Yan WL. Dispersion of graphite nanosheets in a polymer matrix and the conducting property of the nanocomposites. Polym Eng Sci. 2001;41:2148-2154.
- Ezquerra TA, Kulescza M, Balta-Calleja FJ. Electrical transport in polyethylene-graphite composite materials. Synth Met. 1991;41:915-920.
- Brandt W, Berko S, Walker WW. Positronium decay in molecular substances. Phys Rev. 1960;120:1289.
- Jean Y C. Positron annihilation spectroscopy for chemical analysis: A novel probe for microstructural analysis of polymers. Microchem J. 1990;42:72-102.
- Kansy J. Microcomputer program for analysis of positron annihilation lifetime spectra. Nucl Instrum Methods Phys Res Sect A. 1996;374:235-244.
- Liu J, Jean YC, Yang H. Free-volume hole properties of polymer blends probed by positron annihilation spectroscopy: miscibility. Macromolecules. 1995;28:5774-5779.
- Deng Q, Jean YC. Free-volume distribution of an epoxy polymer probed by positron annihilation: Pressure dependence. Macromolecules. 1993;26:30-34.
- Raj JM, Ranganathaiah C. A new method of stabilization and characterization of the interface in binary polymer blends by irradiation: A positron annihilation study. J Polym Sci Part B: Polym Phys. 2009;47:619-632.
- Wästlund C, Berndtsson H, Maurer FH. Miscibility of styrene-maleic anhydride and styrene-acrylonitrile blends studied by positron annihilation lifetime spectroscopy. Macromolecules. 1998;31:3322-3327.
- Ravikumar HB, Ranganathaiah C. Compatibilizer‐induced microstructural changes in poly (Trimethylene terephthalate)/EPDM blends studied by the positron annihilation lifetime technique and differential scanning calorimetry. Polym Int. 2005;54:1288-1295.
- Abd-El Salam MH, El-Gamal S, Abd El-Maqsoud DM, Mohsen M. Correlation of electrical and swelling properties with nano free-volume structure of conductive silicone rubber composites. Polym Compos. 2013;34:2105-2115.
- Patnaik A, Patnaik A, Zhu Z, Yang G, Sun Y. Positron lifetime as a nanoprobe for free volume distribution in High Density Polyethylene-Carbon Black Conducting Composites. Physica Status Solidi (a). 1998;169:115-125.
- Xanthos M. Mica flakes. Functional fillers for plastics. 2005;149.
- Ward AA, Khalaf AI, Ismail MN, Tawfik SY, Mansour SH. Graphite and copper nano-particles in PVC/NBR Composites. Kautschuk Gummi Kunststoffe. 2013;66:36-45.
Citation: Gomaa E, Hassan Ali E (2019) Effect of Graphite and Copper Nano-Particles on Free Volume Properties of PVC/NBR Blends Studied by Pal Spectroscopy. Mod Chem Appl 7:269. doi: 10.35248/2329-6798.19.7.269
Copyright: © 2019 Gomaa E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


