PMC/PubMed Indexed Articles
Indexed In
- Academic Journals Database
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 12, Issue 2
Diagnostic oral biomarkers of immunosuppression in apparently healthy seropositive HIV population, in South Western Uganda
Agwu Ezera*2Department of Microbiology and Immunology, Faculty of Biomedical Sciences, Kampala International University, Box 71, Bushenyi,, Uganda
Received: 13-Dec-2019 Published: 20-Apr-2020, DOI: 10.35248/1948-5948.20.12.429
Abstract
Background: Accurate diagnosis remain key to effective intervention of endemic and pandemic diseases even up to the developed world. Despite availability of many high-quality diagnostic tests for immunosuppression in the developed countries, they are neither available, affordable nor accessible in rural communities of Africa. Clinical diagnostic surrogate biomarkers may be suitable alternative.
Objective: To evaluate oral clinical manifestations as biomarkers of immunosuppression in apparently healthy population of Human Immunodeficiency Virus (HIV) infected patients in resource poor Masaka, Mbarara and Rukungiri districts, of South Western Uganda.
Methods: Visual oral inspection of 304 apparently health and HIV seropositive patients attending the AIDS Support Organization clinics in study districts of Uganda was done to detect and establish oral biomarkers associated with immunosuppression in HIV disease. Standard methods were used to reconfirm the HIV sero-positivity status and clinical staging of oral manifestations of consenting clients.
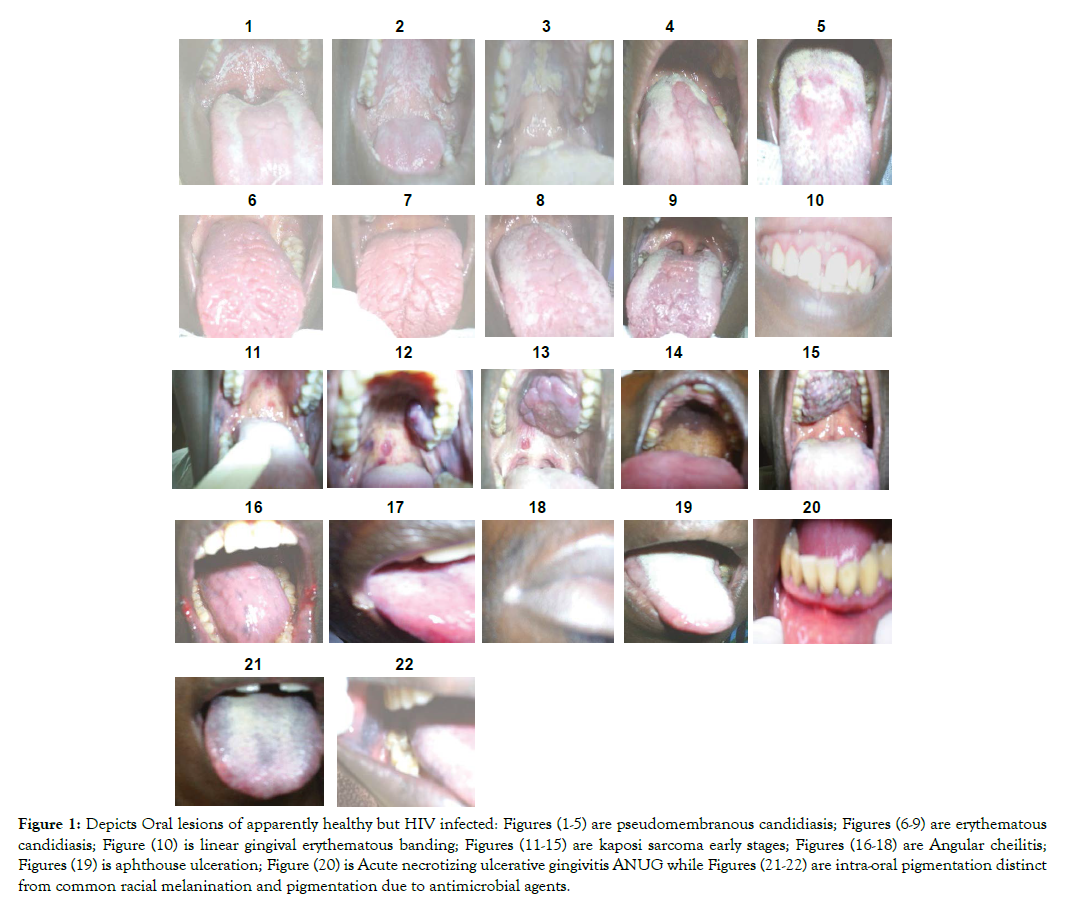
Result: Figures 1-22 shows representative 304 oral manifestations of research participants. Figures 1-5 depicts 140 (46.1%) pseudomembranous candidiasis. Figures (6-9) depicts representative photographs of 53 (17.4%) erythemathous candidiasis (Figures 7 & 8) and also 63 (20.7%) shows erythemathous candidiasis found co-infecting with pseudomembranouse candidiasis in (Figures 9 & 10). Figure 10 shows linear gingival erythemathouse banding. Figures 11-15 shows Karposi sarcoma developmental stages and Figure 16-18 shows 7 (2.3%) Angular cheilitis. Figure 19 shows 3 (1.0%) aphthous ulceration of anterior portion of the tongue. Figure 20 shows 1 (0.3%) Acute necrotizing ulcerative gingivitis (ANUG) while Figures 21 & 22 shows 10 (3.3%) intra-oral pigmentation.
Conclusion: Visual oral inspection of apparently healthy HIV seropositive individuals revealed different oral manifestations that may serve as diagnostic oral biomarkers of immunosuppression in apparently healthy but HIV infected population in Uganda. Poor resources drive the need for available and affordable diagnostic tools for improved and effective intervention.
Keywords
AIDS, Microbiology, Biomarkers, Immunosuppression, HIV, South Western Uganda, Disease Management, Clinical manifestation
Introduction
A biomarker may be defined as an observable characteristic that can be accurately measured and assessed as a pointer or predictor [1] of: Normal biological processes, clinical manifestation [2,3], pathogenic processes [4] or pharmacological responses to a therapeutic intervention [5]. The use of biomarkers in diseases management, to determine new treatment outcome or new strategies of clinical management [6], may be based on recent advances in molecular sciences and imaging techniques. In the active search for new biomarkers, many potential candidates can be considered side by side in a randomized battery of experiments which may eventually yield few positive results [7]. Search for biomarkers is a continuous process involving de novo investigations capable of a paradigm shift in current approaches to human health philosophies. In resource poor settings, use of clinical biomarkers in disease management may be better than basing management of disease on presumptions with no scientific backing. This is because biomarkers can indicate health, pathology, or response to treatment, including unwanted side effects and can act as surrogates or substitutes for clinically meaningful endpoints [8]. Biomarkers of disease can also be diagnostic or prognostic, including capacity to quantify the risk of developing a certain disease, surrogate diagnostic biomarkers can serve as tool to improve the standard of care and reduce the costs of diagnosis [1,4].
Although many resources limited African countries have scaled up the fight against chronic endemic debilitating diseases such as HIV and associated opportunistic infections impacting on their morbidity and mortality database [9,10], significant number of people still fall sick, deteriorate and die without proper care. This may be due to poor health resources and limited diagnostic services with its obvious impact of increasing the number of populations with an unknown disease (HIV) status. Every country has got its own strategy to ensure available health resources are distributed even to hard-to-reach local rural communities [11]. One common uphill task is to provide sustainable health facilities in remote rural communities so that the few experts or professionals that may decide to work there will see the basic settings to help the sick people. Effective disease diagnosis remains the bedrock of effective intervention thereby making diagnostic facilities key to effective healthcare delivery services up to the extent of being ready to face outbreaks.
There is extensive literature about oral microbial lesions and manifestations associated with HIV infections in all age groups. Going by the magnitude of devastation associated with over 5 decades of Human Immunodeficiency Virus (HIV) infection, the need for surveillance to update existing database regarding HIV disease diagnosis cannot be overemphasized especially in resource limited and hard-to-reach local communities of Africa. Delineation and mastery of oral manifestations as surrogate diagnostic biomarkers by all/many cadres of healthcare service providers in rural had-to-reach and resource poor local communities may impact on the capacity to effective role-back the over 5 decadelong sustained escalation of HIV pandemic in many developing countries [12,13]. Although many HIV/AIDS diagnostic tests are available in many cities, they are neither available, affordable nor accessible to healthcare services providers in rural communities of developing countries. This study was designed to describe in details all oral manifestation and signs which represents surrogate diagnostic biomarkers of immune suppression in apparently healthy but HIV infected population seeking healthcare in The AIDS Support Organization (TASO) centers of South Western Uganda. The ultimate goal is to update the existing database of surrogate diagnostic biomarkers by showing that careful visual inspection of the oropharynx for surrogate diagnostic biomarkers (clinical signs and manifestations) of immunosuppression can impact the management and prognostic outcome of chronic devastating diseases such as HIV/AIDS.
Materials and Methods
Sample area, size and inclusion criteria
Three main centers of The AIDS Support Organization “TASO”, located in three districts (Masaka, Rukunguri and Mbarara), and five outreach TASO centers (Katungu, Motoma, Kigarama, Ibwanda and Nyihanga), located in two districts (Bushenyi and Mbarara) all-in South-Western Uganda were used for this investigation. The pilot study which gave rise to this study lasted for 3 weeks one week for each of the 3 main TASO clinics in South Western Uganda. This study took place between July and December 2006 where data was collected, analysed and stored.
The inclusion criterion for oral manifestations as candidate biomarkers in this study were that oral signs and manifestations should be: Visible early before any histopathological changes; indicative after active disease; sensitive, and should correlate with the severity of pathological outcome; accessible in the peripheral tissue; analytically stable in tissue so it can be measured; translational across species; associated with a known diseases mechanism and be can localize immune pathology. Consenting participants included TASO clients: currently registered and known HIV infected client above 18 years of age; have tested positive to HIV using an Enzyme Linked Immunosorbent Assay (ELISA) and any other immunoserological method; have had his/her HIV infection clinically staged not earlier than two weeks prior to time of sample collection and be apparently physically healthy.
A total of 304 (235 females and 69 males) patients with different oral lesions consented to participate in this study and were therefore recruited. This 304 sample size surveyed was guided by the upper limit required to give 95% level of confidence at an expected prevalence of about 74% [14] using the precise prevalence formula: Sample size (N)= Z2P (100-P)/D2 giving 296 samples, (Epi-info version 3.2 data-base; 1995), where Z is a constant given as (1.96), P is expected prevalence (74%) [14], and D is acceptable error (5%). Eight more samples were added arbitrarily just in case of drop out and unseen error making a total of 304 samples. Informed consent was sought and obtained from the following: Uganda National Council of Science and Technology, Kampala International University Research and Ethics Committee, The AIDS Support Organizations (TASO) at both local and National level and TASO clients (patients) through their informed consent.
Mouth examination and sample collection: The diagnostic criterion for the oral lesion was according to World Health Organization’s established clinical criteria for HIV-associated oral lesions. The mouth examination of the TASO clients was done by a previously trained and calibrated [14] oral clinicians which included a clinical Microbiologist assisted by a retired dental surgeon and, a public dental health officer.
Prior to field survey, the Clinical Microbiologist and assistant Dental Surgeon, were standardized for consistencies in examination of oral lesions to minimize inter examiners variability using the guidelines adopted in our earlier report [14]. Briefly, a gold standard (benchmark) examiner showed the examiners some photographic slides with clinical examples of classical oral signs and manifestations expected to be seen during the study. This was done to determine the examiners’ ability to uniformly identify and report oral manifestations and signs of immunosuppression with minimum inconsistency. The apparently health but HIV infected clients were examined in a room with a natural light while seated on an office chair and facing the window. The mouth was examined using natural light and a mouth mirror. A disposable wooden tongue depressor was used to retract the cheeks. Some clinical photographs of classical examples of the lesions were taken using digital camera.
Results
Table 1 and Figures 1-22 below shows representative 304 oral manifestations and signs of apparently healthy but HIV infected patients who participated in this study. Figures 1-5 depicts representative photographs of 140 (46.1%) pseudomembranous candidiasis. Figures (6-9) depicts representative photographs of 53 (17.4%) erythemathous candidiasis (Figures 6 & 7) and also 63 (20.7%) shows erythemathous candidiasis found co-infecting with pseudomembranouse candidiasis in (Figures 8 & 9). Figure 10 shows linear gingival erythemathouse banding. Figures 11-15 shows Karposi sarcoma developmental stages and Figures 16-18 shows 7 (2.3%) Angular cheilitis. Figure 19 shows 3 (1. 0%) aphthouse ulceration of anterior portion of the tongue. Figure 20 shows 1 (0.3%) Acute necrotizing ulcerative gingivitis ANUG while Figures 21 &22 shows 10 (3.3%) intra-oral pigmentation.
| Oral Lesions | n=304 |
|---|---|
| Number (%) positive | |
| No Exam | |
| Pseudo Candidiasis | 140 (46.1) |
| Angular Chelitis | 7 (2.3) |
| Eryth Candidiasis | 53 (17.4) |
| Kaposi sarcoma | 9 (3.0) |
| Rec Apth Ulceration | 3 (1. 0) |
| ANUG | 1 (0.3) |
| Pseudo & Eryth Cand | 63 (20.7) |
| Pseudo & Angular | 18 (5.9) |
| IOP | 10 (3.3) |
n = Total number of population sampled, Rec Apth Ulceration = Recurrent Apthous Ulceration, ANUG = Acute Necrotizing Ulcerative Gingivities, Eryth = Erythematous, Pseudo = Pseudomembranous. No Exam=Number examined. IOP = Intra-Oral Pigmentation
Table 1: Study of prevalence of oral lesion among the 304 HIV/AIDS patients.
Figure 1: Depicts Oral lesions of apparently healthy but HIV infected: Figures (1-5) are pseudomembranous candidiasis; Figures (6-9) are erythematous candidiasis; Figure (10) is linear gingival erythematous banding; Figures (11-15) are kaposi sarcoma early stages; Figures (16-18) are Angular cheilitis; Figures (19) is aphthouse ulceration; Figure (20) is Acute necrotizing ulcerative gingivitis ANUG while Figures (21-22) are intra-oral pigmentation distinct from common racial melanination and pigmentation due to antimicrobial agents.
Discussion
Though it may be difficult to define a diagnostic biomarker which has all possible characteristics, oral manifestation or signs recorded as diagnostic biomarkers in this study possessed the following qualities: Visible and suggestive early in disease process before onset of any histopathological changes; sensitive and correlates with the severity of immune pathology; accessible in the peripheral tissue; measurable and analytically stable in tissue; translational across species; associated with known disease mechanism and localize disease outcome [1,4]. HIV infection in sub-Saharan Africa remain a challenge because regional development and welfare depend upon measuring the impact of mortality and morbidity in those already infected with diagnostic tools that is costly, unavailable and inaccessible. Among the HIV infected population, those with unknown HIV status pose danger to the community because they may not avoid risky HIV transmission behaviours thereby contributing significantly to HIV transmission [15]. Although use of anti-retroviral therapy may present with chemotherapeutic symptoms and signs which needs monitoring with laboratory investigation, screening the population with reported surrogate diagnostic alternatives is still better than nothing [16,17]. Despite this limitation, the need for, and use of surrogate diagnostic biomarkers can still be re-emphasized as key to the feasibility of voluntary testing and counselling in hard-to reach communities, helping TASO to reach out to greater percentage of the teaming population of old and new HIV patients at the grass root [16-19].
Because many TASO patients are located in remote villages where services are rare with no laboratory services, need for cheap, available accessible relevant diagnostic alternative candidate surrogate biomarkers that would define the signature pattern of early immunosuppressive. Competency in the use of mere visual inspection of the mouth to diagnose immunosuppression remain promising and could be the answer to diagnosis in resource limited rural settings [7]. AIDS defining opportunistic infections heralds the emergence of HIV immunosuppression but changing diseases epidemiological factors demands use of tools that will offer more diagnostic precision in resources poor [20-22]. Even if this study is taken as one of the many sporadic reports about oral manifestation in African HIV disease, putting the observations in the context of biomarkers will encourage clinicians and research scientist as more questions and get more answers for the better health of the patients. Thus, in-depth descriptive analysis of various oral manifestations prevalent among consenting apparently health but HIV infected participants may usher in a new diagnostic horizon with use of surrogate markers of immunosuppression in unknown HIV population [8,23]. Oral manifestation is known prognostic markers of: HIV infection, disease progression, selected AIDSrelated immunosuppressive illnesses, the decline in numbers of CD4+ cells as well as the corresponding increase in viral load [24- 26].
In (Figures1-5), diagnosed as Pseudomembranous Candidiasis (PC), observed presence of removable creamy whitish plaques on all the oral mucosal surfaces may be associated with initial and progressive immune deterioration represented by CD4<400 and the white non removable plaque seen in Figure 3, may be associated with severe immune suppression and risk for oral carcinoma [27,28]. A rare observation of esophageal Candidosis pattern was seen in Figure 1 showing entire uvula, soft palate and tonsils being affected and may depict tendencies to cause uvulitis and tonsillitis (Figure 1). Based on duration of HIV sero-positivity, three unique types of PC manifestation stood out. Among consenting 50.2% of group one patients who knew their HIV status for 2 years, PC covered the entire dorsum of the tongue concentrating more on central sulcus (towards the posterior one third) which mark the fusion of the tongue during development, (Figure 5). In group two found among 41.0% of the patients who knew their status for 2-7years PC concentrated on the periphery of the mouth dorsum leaving out the median plain where the central sulcus is located (Figure 4). In group three, found among 6.6% of patients who knew their status for 7-12years, PC left the median plain, was less on the periphery and margins of the tongue, but concentrated on the posterior one third of tongue dorsum and heaviest on oropharynx (Figures 1 & 5). It was not clear why PC which covered entire tongue dorsum left a unique central margin where the vallate papillae are located (Figure 5). Further test using Immuno-Histo-Chemical technology is recommended to see if more information on the histology, Immunology and Chemistry of the location can be obtained.
Although Erythematous Candidiasis (EC), which supposed to represent early HIV infection, is underreported due to misdiagnosis or under diagnoses, (Figures 6-9) may then represent classical manifestation of EC in apparently healthy but HIV infected population in Uganda [29]. This study reveals EC being predominant in both early and late disease onset. Thus, the EC was very conspicuous, characteristically removed of all the oral mucous membranes exposing the muscles of the tongue but not the hard/soft palates [28,29]. Patients with EC mostly complained of no taste when eating normal food, oral burning, while eating salty or spicy foods or drinking acidic beverages. It is not common to see people live with HIV for 10 years and still remain apparently healthy outside when the mouth is systematically dilapidated. Again, a close look at the 50.2% of patients who knew their HIV status for 2 years, showed that patients had EC which covered the central dorsum of the tongue where the central sulcus is located leaving PC at the margin of tongue (Figures 8 & 9). The 41.0% of the patients who knew their status for 2-7years, had also had EC found on the anterior two third of tongue dorsum, having removed the mucous covering of the tongue exposing the tongue muscles (Figure 7). Again 6.6% of patients who knew their status for 7-12years, had EC which had become severe in the entire tongue dorsum (Figures 6 & 7), and later succeeded in creating a median furrow along the central sulcus, which marked the fusion of the two halves of the tongue during development.
An individual, fiery, red band along the margin of the gingiva, seen on the anterior teeth (Figure10), was identified as linear gingival erythematous banding [27,28,30]. While the significance of linear gingival erythematous banding as biomarker of HIV infection is being debated with respect to its existence in non-HIV-infected population; and whether it is restricted to adult infected HIV patients, efforts should be made to confirm or rule out its diagnosis in the context of underlying immunosuppression.
Two distinct types of oral Kaposi sarcoma mass were observed among the apparently health but HIV infected and consenting participants. Smooth segmented lobe-like mass (Figure 13) and rough continuous none segmented mass (Figure 15), seen among those who knew their status for 7-12 years (Figures 11-15). Smooth segmented lobe-like mass may have emerged from red spots on the palates (Figure 11) because of the observed red spots along with smaller smooth mass (Figure 12) and red spots found along side with the Smooth segmented lobe-like mass (Figure 13). The rough continuous non segmented mass (Figure 15) may have emerged from brown to black pigmented palate (Figure 14), though smaller mass was not seen among the studied participants. Red spots and brown pigmentation were seen among patients who knew their status for up to 2 years and red spots with smaller mass was seen among participants who knew their status for 2-7 years. The rough mass emerging from the brown or black pigmentation were not seen among those who had lived with the virus for 2-7 years. This observation indicates immune deterioration among apparently healthy but HIV infected population. All patients with similar lesions like those in (Figures 11-15) were referred for histological confirmation as KS or non-Hodgkin’s lymphoma and for adequate therapy. It was not clear why KS were most prevalent in one village (Ibwanda) located in Mbarara District. A follow-up study is therefore recommended to determine the possible risk factors. Cross-sectional studies have associated low CD4+ lymphocyte counts with the presence of oral Kaposi sarcoma [30] and even non- Hodgkin lymphoma [31] in cases where KS has been differentiated from non-Hodgkin lymphoma making both condition suitable surrogate biomarkers for immune suppression in apparently health population.
Angular Chelitis (AC) presented as erythema and fissuring of the corners of the mouth was common in all stages of HIV infection (Figures 16-18), similar to the published reports [28,32]. Recurrent Aphthous Ulceration (RAU); Figure-19 presented as painful ulcers on the non-keratinized oral mucosa, soft palate, and ventral aspect of the tongue. Pain due to RAU was mostly noted to increase upon eating salty, spicy or acidic foods and beverages as well as due to trauma when consuming hard or rough foods. Acute Necrotizing Ulcerative Gingivitis (ANUG) presented as painful necrotic ulcers of the tip of inter dental papillae, spreading along the marginal gingiva and affecting soft tissues only (Figure 20). It is reported to be less severe than Necrotizing Ulcerative Gingivitis (NUG) and may be induced by stress or poor diet [33]. Cross sectional studies have been reported which related low CD4 cell count with the presence of this type of lesion [30] making both ANUG a suitable surrogate biomarker for immune suppression in apparently health population.
Intra-Oral Pigmentations (IOP); (Figures 21 & 22), a rarely reported manifestation in this region were seen presenting on the entire tongue dorsum, margin, cheeks, posterior one third and anterior two third of the tongue (Figures 21 & 22) [34-36]. Ranganathan et al., [37] reported in South India that its occurrence is possibly due to increased melanin pigmentation in skin and oral mucosa. Some of the reasons that have been put forward to explain the intraoral pigmentation especially in HIV infection are increased release of α-melanocyte stimulating hormone (α-MSH) due to deregulated release of cytokines in HIV disease; use of melanocyte stimulating drugs: Certain antiretroviral, antifungals and Addison’s disease [33,34,37]. In South Western Uganda, it is thought that this form of pigmentation is genetic and therefore inherited from birth.
The observation of 40.9% mixed infection of PC with other oral lesions especially among people living with HIV for longer period up to 12 years might suggest possible need for correlation with immune deterioration an observation that needs further studies equivalent to known CD4+ correlation studies with oral lesions. However, these defects may be partly compensated by preserved host defense mechanisms (calprotectin, keratinocytes, CD8+ T cells, and phagocytes) which, individually or together, may limit Candida albicans proliferation to the superficial mucosa [38].
The known evidences suggest a number of hypothetical defects which may underlie the susceptibility to mucosal candidiasis in HIVinfection.. These include the fact that depletion of Langerhans' cells and their reduced expression of Major Histocompatibility Cells (MHC) class II molecules and IL-12 probably perturb the development of Candida-specific CD4+ Th1 cells which are instrumental in orchestrating a protective adaptive cell-mediated immune response to C. albicans in the oral and esophageal mucosa [39]. In addition, the depletion of CD4+ cells and a shift in expression from Th1 to Th2 cytokines may reduce the anti-candidal activity of macrophages and PMNs and thus trigger the onset of OPC. Partly, preserved expression of MHC class I antigens on Langerhans' cells may also allow the recruitment of a compensatory protective CD8+ T-cell response to C. albicans in the mucosa despite HIV infection; this, combined with the anti-candidal activity of preserved innate defense mechanisms (calprotectin and keratinocytes), may limit the proliferation of C. albicans to the mucosa and prevent systemic dissemination to deep organs [29,39].
Oral Keratinocytes are of primary importance in the pathogenesis of OPC since they: constitute a physical barrier; function as fixed or immobile immunocytes; are capable of producing a number of factors (TNF-α, IL-1, IL-3, IL-6, etc.), involved in up-regulating and down-regulating immune responses and express the adhesion molecules CD54 and CD58 [39]. Oral epithelial keratinocytes are thus equipped with numerous redundant defense mechanisms, acting either directly or indirectly against the continuous microbial challenge at the oral mucosal surface. The role of keratinocytes in host protection against Candida at mucosal surfaces appears likely, since C. albicans hyphae are restricted to the upper layers of the oral epithelium in OPC and are some distance away from lymphocytes and Langerhans' cells located in deeper layers [33,34].
Conclusion
In conclusion, oropharyngeal manifestations reported in this study matched what other researchers had reported elsewhere as indicators of immunosuppression and they also qualify for use as surrogate biomarkers of immune suppression in remote hard to reach settings of resource poor Countries. Updated correlation studies of observed biomarkers with CD4 cells and with pattern of disease comorbidities are highly recommended.
Data Availability
All data used for this investigation have been included within the text including tables and figure and they are available for use without any restrictions. The pictures are original and terms of use are according to the terms of publication journal or house if the paper is accepted for publication.
Conflict of Interest
None declared.
Sponsorship
This study was sponsored in part by the AIDS support organization (TASO) Uganda, and Kampala International University.
Acknowledgement
The data for this manuscript was collected in six months between July and December 2006. The pilot for this study was the preliminary result of this manuscript and was presented as one paragraph abstract “Oral predictors of immunosuppression among apparently health population of HIV infected patients in south western Uganda” at the 2nd International Conference on Clinical Microbiology & Microbial Genomics, September 16-17, 2013 Hampton Inn Tropicana, Las Vegas, NV, USA. The attendees of the conference confirmed the originality of this work despite the delay in publishing the data. TASO management need this paper as guide to diagnosis in rural settings.
Dr. Francis Tirwomwe and Mr. Michael Tirwomwe are hereby acknowledged for their clinical assistance during this study.
REFERENCES
- Strimbu K, Tavel JA. What are Biomarkers? Curr Opin HIV AIDS. 2010;5:463-466.
- Begg MD, Panageas KS, Mitchell-Lewis D, Bucklan RS, Phelan JA, Lamster IB. Oral lesions as markers of severe immunosuppression in HIV-infected homosexual men and injection drug users. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996, 82:276-283
- International AIDS Society (IAS)–USA. Topics in HIV Medicine Oral Manifestations - Most misdiagnosed or underdiagnosed. Perspective – Oral Manifestations 2005,13(5)
- Ackerman H, Casals-Pascual C. A biomarker approach to syndrome-based treatment of severe childhood illness in malaria-endemic areas. Malar J. 2018;17:378.
- Wieland E, Olbricht CJ, Susal C, Gurragchaa P, Bohler T, Israeli M, et al. Biomarkers as a tool for management of immunosuppression in transplant patients. Ther Drug Monit. 2010;32:560-572.
- Zecevic L, Karamehic J, Coric J, Stubljar D, Avdagic N, Selmanovic K, et al. Potential immune biomarkers in diagnosis and clinical management for systemic lupus erythematosus. J Med Biochem. 2018;37:163-171.
- Saxena D, Li Y, Devota A, Pushalkar S, Abrams W, Barber C, et al. Modulation of the orodigestive tract microbiome in HIV-infected patients. Oral Dis. 2016;22:73-78.
- Fleming TR, Powers JH. Biomarkers and surrogate endpoints in clinical trials. Stat Med. 2012 Nov 10;31(25):2973-84.
- Baluku M, Agwu E, Kasule A, Moazzam ML. The transmission dynamics of cholera epidemic in Kasese district, Uganda. Special Path Rev J. 2015;1:30-39.
- Agwu E, Ihongbe JC, Ezeonwumelu JO, Lodhi MM. Baseline burden and antimicrobial susceptibility of pathogenic bacteria recovered from oral lesions of HIV/AIDS patients in South-Western Uganda. Oral Sci Int. 2015;12:59-66.
- Kasule A, Agwu E. Review of health workers attrition and microbial disease endemicity in Bushenyi, Ntungamo and Rukungiri Districts of South Western Uganda. Spec Pathog Rev J, 2015;1:1-15.
- Ihongbe JC, Moazzam ML, Pazos V, Agwu E. Non-target oral bacterial resistance to Cotrimoxazole in HIV/AIDS patients living in South Western Uganda, Special Bact Pathog J. 2015;1:1-4.
- E. Agwu, V. Pazos, J. C., Ihongbe , J. Ssengendo. Appraisal of the inherent socio-demographic dynamics of HIV/AIDS epidemic in four districts of South-Western Uganda. Sahara J. 2011;8:150-155.
- Agwu E, Ihongbe JC, Tirwomwe JF, Pazos V, Tirwomwe M, Casadesus L. Appraisal of Oral Lesions status of HIV/AIDS Patients in South Western Uganda” Br J Oral Sci. 2008;7:1591-1595.
- Coates TJ, Richter L, Caceres C. Behavioural strategies to reduce HIV transmission: How to make them work better. Lancet. 2008;23:669-684.
- Tamí-Maury IM, Willig JH, Jolly PE, Vermund S, Aban I, Hill JD, et al. Prevalence, incidence, and recurrence of oral lesions among HIV-infected patients on HAART in Alabama: A two-year longitudinal study. South Med J. 2011;104:561-566.
- Perera M, Tsang PC, Samaranayake L, Lee MP, Li P. Prevalence of oral mucosal lesions in adults undergoing highly active antiretroviral therapy in Hong Kong. J Investig Clin Dent. 2012;3:208-214.
- Nielsen H, Bentsen KD, Hojtved L, Willemoes EH, Scheutz F, Schiodt M, et al. Oral candidiasis and immune status of HIV-infected patients. J Oral Pathol Med. 1994;23:140-143.
- F. J. Ramos-Gomez, S. L. Tomar, J. Ellison, N. Artiga, J. Sintes , G. Vicuna. Assessment of early childhood caries and dietary habits in a population of migrant Hispanic children in Stockton, California. ASDC J Dent Child. 1999;66:395-403, 366.
- C. H. Shiboski, J. A. Regezi, H. C. Sanchez and S. (Jr) Silverman. Oral lesions as the first manifestation of microscopic polyangiitis: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:707-711.
- E. Agwu, S. Oming, M. L. Moazzam. Prevalence of Cryptosporidiosis among diarrhea patients attending clinics in Bushenyi district of Uganda. Spl Parasites Pathol J. 2015;l:14-19.
- Agwu E. Incidence of Streptococcus pneumoniae Infections Among Patients Attending Tuberculosis Clinics in Ekpoma, Nigeria. Shiraz E-Med J. 2006;7:20395.
- Lifson AR, Hilton JF, Westenhouse JL, Canchola AJ, Samuel MC, Katz MH, et al. Time from HIV seroconversion to oral candidiasis or hairy leukoplakia among homosexual and bisexual men enrolled in three prospective cohorts. AIDS. 1994;8:73-79.
- Greenspan JS, Greenspan D. The epidemiology of the oral lesions of HIV infection in the developed world. Oral Dis. 2002;8:34-39.
- Revankar SG, Sanche SE, Dib OP, Caceres M, Patterson TF. Effect of highly active antiretroviral therapy on recurrent oropharyngeal candidiasis in HIV- infected patients. AIDS. 1998;12:2511-2513.
- Kamulegeya A. Maxillofacial sarcomas: A Ugandan epidemiological survey. O J Stomatology. 2011;1:50-54.
- Mary EO, Abiola OA, Titilola G, Mojirayo OO, Sulaimon AA. Prevalence of HIV related oral lesions in people living with HIV and on combined antiretroviral therapy: A Nigerian experience. Pan Afr Med J.2018;31:180.
- Sirosis DA. Oral manifestation of HIV Disease. Mt Sinai J Med. 1998;65:322-332.
- Challacombe SJ. Global oral inequalities in HIV infection. Oral Dis. 2016;22:35-41.
- Axell T, Azuuln AM, Challacombet SJ, Fiarra G. Flint S, Grenspan D et al. Classification and diagnostic criteria for oral lesions in HIV infection. EC-Clearinghouse on oral problems related to HIV infection and WHO Collaborating Centre on oral manifestations of the human immunodeficiency virus. J Oral Pathol Med. 1993;22:289-291.
- Glick M, Muzyka BC, Lurie D, Salkin LM.. Oral manifestations associated with HIV-related disease as markers for immune suppression and AIDS. Oral Surg Oral Med Oral Pathol. 1994;77:344-349.
- Flaitz CM, Nichols CM, Hicks MJ. Oral malignancies diagnosed in an HIV- dedicated dental clinic. Tex Dent J. 1996;113:49-57.
- Robinson PG. The significance and management of periodontal lesions in HIV infections. Oral Dis. 2002;8:91-97.
- Chandran R, Feller L, Lemmer J, Khammissa RA. HIV-associated oral mucosal melanin hyperpigmentation: A clinical study in a South African population sample. AIDS Res Treat. 2016;2016:8389214.
- Feller L, Chandran R, Kramer B, Khammissa RA, Altini M, Lemmer J. Melanocyte Biology and Function with Reference to Oral Melanin Hyperpigmentation in HIV-Seropositive Subjects. AIDS Res Hum Retroviruses. 2014;30:837-843.
- Tatfeng YM, Nwobu GO, Okokua MA, Okogun GRA, AgbaMI, Agwu E. Effects of Lamivudine (Epivir), Nevarapine (Vivumin) and Stavudine (Stavir) combination on CD4+ count of HIV patients. Kuwait Med J. 2005;37:86-90.
- Ranganathan K, Umadevi M, Saraswathi TR, Kumarasamy N, Solomon S, Johnson N. Oral lesions and conditions associated with human immunodeficiency virus infection in 1000 South Indian patients. Ann Acad Med Singapore. 2004;33:37-42.
- Moyes DL, Saxena D, John MD, Malamud D. The gut and oral microbiome in HIV disease: a workshop report. Oral Dis. 2016;22:166.
- de Repentigny L, Lewandowski D, Jolicoeur P. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clin Microbiol Rev. 2004;17:729-59.
Citation: Ezera A (2020) Diagnostic oral biomarkers of immunosuppression in apparently healthy seropositive HIV population, in South Western Uganda. J Microb Biochem Technol. 12:429. 5:162 doi: 10.35248/1948-5948.20.12.429
Copyright: © Ezera A. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Competing interests: The authors have declared that no competing interests exist.