Research - (2021) Volume 9, Issue 6
The environmental friendly Pyrazole carbohydrazideinhibitor is synthesized and their corrosion inhibition for AA 8088in a 1 M H2SO4 solution was studied using weight loss methods, electrochemical measurements, and the surface morphology of AA 8088with and without inhibitor were studied using scanning electron microscopy (SEM) analysis. The inhibition efficiency of 1-(4-Methoxy-phenyl)-3-(5-phenyl-1, 3, 4-oxadiazol-2-yl) propan-1-one improved with increases in inhibitor concentration but decreased with increases in temperature. Results from potentiodynamic polarization and EIS showed that the corrosion inhibition efficiency of Pyrazole carbohydrazidewas excellent. Morphology observation revealed that the AA 8088was greatly protected by these Pyrazole carbohydrazideinhibitor.
AA 8088; Potentiodynamic polarization; Corrosion inhibition
Corrosion of aluminum and its alloys has attracted much attention from many researchers due to their high mechanical intensity, low cost, low density, and good machinability, and they have been widely used in industrial applications, especially in constructions, electronics, packing, storage, and transportation equipment and machinery [1–6]. Corrosion is an electrochemical process and is often activated by industrial processes such as acid descaling, acid pickling, acid cleaning, and oil well acidizing [7]. Efforts have been made to protect the integrity of the aluminum surface in an aggressive acid medium or other corrosive environment. In recent decades, the addition of inhibitors has been considered to be the most common approach to hinder the corrosion of aluminum [8–10].
Many organic compounds have been widely reported as corrosion inhibitors of aluminum in acid solution, such as aliphatic, aromatic amines, and nitrogen heterocyclic molecules [11–15]. However, some of these compounds are costly and not easily biodegradable. As high reactive, low cost, high solubility, and environmentally friendly compounds, triazinedithiol, and its monosodium salt have been reported to prepare the effective corrosion inhibitive film on metal surfaces by electrochemical deposition [16–19]. The special tautomer of thiol–thione with highly electronegative atoms like S and O, and the N-containing heterocyclic conjugate system, benefit the Pyrazole carbohydrazidemolecules to adsorb on metallic surface. However, the research on Pyrazole carbohydrazideinhibitors for AA 8088is seldom reported. The purpose of present work is to investigate and compare the corrosion inhibition action of Pyrazole carbohydrazideand their protective performance for AA 8088(AA 8088) in 1 M H2SO4 was studied utilizing a variety of electrochemical tests, weight loss methods, scanning electron microscopy (SEM) techniques.
Materials and sample preparation
The AA 8088sheet having chemical composition is as follows: MS was mechanically press cut into specimens of dimension 30 mm × 50 mm × 0.3 mm. All test plates of AA 8088were ultrasonically degreased in the acetone for 15 min, and treated by the immersion in alkaline solution (15 g Na2CO3+15 g Na2PO4 per liter) at 60°C for the debinding process [20]. After that, the AA 8088specimens were washed thoroughly with distilled water and dried with nitrogen. The specimens with an exposed area of 1 cm2 were used for potentiodynamic polarization and electrochemical impedance spectroscopy. AR (analytical reagent) grade hydrochloric acid and double distilled water were used to prepare the corrosive media. In this paper, Synthesis of Pyrazole carbohydrazideAryl hydrazide (1 M) was dissolved in phosphorous oxychloride (5 mL) the reaction mixture, after refluxing for 6-7 hours, was cooled to room temperature and poured onto crushed ice. On neutralization of the contents with sodium bicarbonate solution (20%), a solid mass separated out. This was filtered and washed with water. It was crystallized by using methanol Pyrazole carbohydrazideand Yield 89.90%, yellow powder, mp (°C): 240– 242 (Table 1).
| Chemical composition | |
|---|---|
| Carbon | 0.16-0.18% |
| Silicon | 0.40% max |
| Manganese | 0.70-0.90% |
| Sulphur | 0.040% Max |
| Phosphorus | 0.040% Max |
Table 1: Chemical Composition of AA 8088.
Electrochemical measurements
The potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) measurements were carried out using CHI 608E electrochemical work station (CHI Instruments; USA) in a three electrode cell system with a saturated calomel electrode (SCE) as reference electrode and a rectangular piece of graphite as counter electrode. The working electrode was AA 8088. Prior to any electrochemical measurements, the immersion in the solution for 1 h was necessary for the open circuit potential to reach a steady state. EIS was carried out at steady open circuit potential disturbed with amplitude of 10 mV alternative current sine wave in the frequency range of 100 MHz to 10 kHz. The polarization curves were obtained by changing potential from -250 mV to +250 mV versus OCP with a scan rate of 0.5 mV/s (Figure 1).

Figure 1: Experimental set up for potentiodynamic and EIS studies
Scanning Electron Microscopy (SEM)
The surfaces morphologies of the AA 8088immersed in a 1 M H2SO4 solution for 2 h with and without the Pyrazole carbohydrazideinhibitors were observed via SEM (JSM-6360LV, JEOL, Tokyo, Japan) at an accelerating voltage of 20 kV, respectively.
Potentiodynamic polarization profiles for AA 8088with different concentrations of Pyrazole carbohydrazideare presented below. The corrosion kinetics parameters such as corrosion potential (Ecorr), corrosion current density (Icorr), and cathodic and anodic Tafel slopes were given in Table 2, where the inhibition efficiency was calculated (Figure 2).
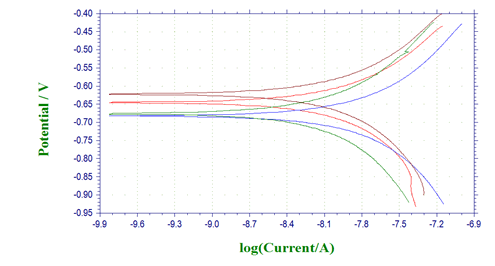
Figure 2: Tafel plots for AA 8088in 1M H2SO4containing different concentrations of 1-(4-Methoxy-phenyl)-3-(5-phenyl-1,3,4-oxadiazol-2-yl)propan-1-one
Compared with the blank solution, the cathodic currents were significantly decreased with the presence of inhibitors and the addition of these compounds made Ecorr shifted towards negative potentials (Figure 2), which suggested that 1-(4-Methoxy-phenyl)- 3-(5-phenyl-1,3,4-oxadiazol-2-yl) propan-1-onegreatly reduced the hydrogen evolution reaction, but their inhibition effects on the anodic dissolution were unobvious. Besides, the addition of 1-(4-Methoxy-phenyl)-3-(5-phenyl-1,3,4-oxadiazol-2-yl) propan- 1-oneshift the cathodic and anodic curves to lower values, while the concentration of inhibitors was increased [21-24] (Table 2).
| Inhibitor | C (ppm) | Ecorr(mV/SCE) | Icorr(µA·cm-2) | �a(mV�dec-1) | �c(mV·dec-1) | np (%) |
|---|---|---|---|---|---|---|
| A1 | Blank | -735.90 | 2852.4 | 118.3 | 90.5 | |
| 50 | -742.10 | 835.3 | 128.3 | 88.7 | 70.72 | |
| 100 | -739.00 | 316.5 | 136.5 | 96.4 | 88.9 | |
| 150 | -740.40 | 251.9 | 140.1 | 90.8 | 90.17 |
Table 2:Tafel polarization parameters of the corrosion for AA 8088in 1M H2SO4containing different concentrations of 1-(4-Methoxy-phenyl)-3-(5-phenyl-1,3,4-oxadiazol-2-yl)propan-1-one.
It is clearly seen that, when more inhibitors were added into the corrosive solution, the corrosion current density decreased and the inhibition efficiency increased. When the concentration of Pyrazole carbohydrazidereached 1 mM, the lowest Icorr values of 63.6 μA.cm2 and 33.5 μA.cm2were obtained, and the inhibition efficiency achieved 97.77% and 98.83%, respectively. Generally, a compound is considered to anodic or cathodic type when the displacement in Ecorr is greater than 85 mV; otherwise, inhibitor is considered as a mixed type [25]. For 1-(4-Methoxy-phenyl)- 3-(5-phenyl-1,3,4-oxadiazol-2-yl) propan-1-one, the Ecorr values shift towards more a negative direction compared with the blank solution, but the change is not significant when the maximum displacement of Ecorr values is 10.9 mV, which indicated that 1-(4-Methoxy-phenyl)-3-(5-phenyl-1,3,4-oxadiazol-2-yl) propan- 1-onebelonged to mixed-type inhibitors, mainly inhibiting the cathodic processes.
Electrochemical Impedance Spectroscopy (EIS)
Nyquist plots for AA 8088in the absence and presence of various concentrations of 1-(4-Methoxy-phenyl)-3-(5-phenyl-1,3,4-oxadiazol- 2-yl) propan-1-oneare given below (Figure 3). The impedance spectra are consisted of capacitive loops at higher frequency and inductive loops at lower frequency. The presence of depressed semicircle in Nyquist plot across the studied frequency range indicates that a charge transfer process mainly controls the corrosion of aluminum. In other literature, similar plots have been reported for the corrosion of aluminum alloys in HCl solutions [20]. The inductive loop is generally attributed to the relaxation process in the oxide film covered on metal surface [26]. The reasons behind the deviations from perfect semicircles are usually involved with the frequency dispersion of interfacial impedance, which can be attributed to various kinds of physical phenomena such as active sites, surface roughness, and non-homogeneity of the solids [27]. The diameter of the capacitive loop is enlarging gradually with increasing concentrations of inhibitor, indicating that the charge transfer resistance is increased and the adsorbed inhibitor forms a more compact monolayer on metal surface with an increasing amount of inhibitor.
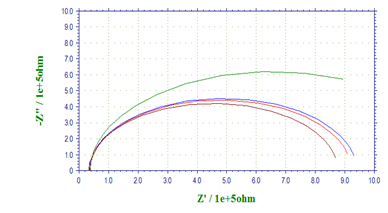
Figure 3: Nyquist diagrams for AA 8088in 1M H2SO4containing different concentrations of 1-(4-Methoxy-phenyl)-3-(5-phenyl-1,3,4-oxadiazol-2-yl)propan-1-one
Surface morphology
SEM technique was employed to further prove the corrosion resistance ability of DAN and DBN, and the surface observation images of AA 8088after a 2 h exposure in a HCl solution without and with inhibitors are shown below (Figure 4). Before immersion, the bare aluminum plate looks very smooth. In contrast, in the absence of inhibitor, the AA 8088presented a very rough surface covered with a huge amount of deep cracks and large holes, which suggests strong damage and a severe dissolution of AA 8088in contact with aggressive solution. Nevertheless the dissolution rate of AA 8088was substantially inhibited by DAN and DBN, exhibiting a comparative smooth surface with a few small pits [28]. Therefore, it is concluded that the regular distribution of the DAN or DBN molecules adsorbed on AA 8088surface generates consistent protective layers, which effectively prevent HCl molecules from penetrating into the aluminum surface [29].
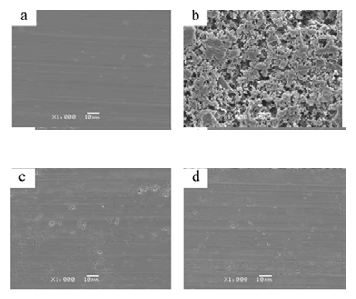
Figure 4: SEM images of AA 8088surface before and after immersing in 1M H2SO4without and with Pyrazole carbohydrazideinhibitor. (a) Blank before immersion; (b) blank after immersion; (c) with 1-(4-Methoxy-phenyl)-3-(5-phenyl-1,3,4-oxadiazol-2-yl)propan-1-one(50ppm) (d) with 1-(4-Methoxy-phenyl)-3-(5-phenyl-1,3,4-oxadiazol-2-yl)propan-1-one(150ppm)
Mechanism of inhibition
Corrosion inhibition of AA 8088in 1 M hydrochloric acid solution byPyrazole carbohydrazideinhibitor can be explained on the basis of molecular adsorption. These compounds inhibit corrosion by controlling both anodic as well as cathodic reactions. In 1 M hydrochloric acid solution this inhibitor exists as protonated species. In the inhibitor the nitrogen atoms present in the molecules can be easily protonated in acidic solution and convert into quaternary compounds. These protonated species adsorbed on the cathodic sites of the AA 8088 and decrease the evolution of hydrogen [30-33].
Pyrazole carbohydrazidecompound as corrosion inhibitors for AA 8088 in a 1 M H2SO4 solution were investigated. For 1-(4-Methoxy-phenyl)-3-(5-phenyl-1,3,4-oxadiazol-2-yl) propan- 1-one, their inhibition efficiency increased with increases in inhibitor concentration and they belonged to mixed-type inhibitors predominantly retarding the cathodic reaction. The inhibiting efficiencies determined by potentiodynamic polarization testing, and EIS measurements are all in good agreement. The surface morphologies images were good proof for the reduction of dissolution of AA 8088ascribed to the formation of protective Pyrazole carbohydrazidefilm on the metal surface.
Received: 26-Jun-2021 Published: 17-Jul-2021, DOI: 10.35248/2329-6836.21.9.407
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.