Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
+44 1300 500008
ISSN: 2155-9880
+44 1300 500008
Review Article - (2021)Volume 12, Issue 7
Background: Congenitally corrected transposition of the great arteries (CCTGA) is a rare complex cardiac anomaly with a unique anatomical lesion such that despite certain anatomical aberrations there could be physiologically normal circulation without any admixture. They could also be anatomical lesions and various clinical evolutions. Surgical intervention has continued to change and updated.
Methods: A search for published works on ccTGA was made using Google and Pub Med as well as searching through the Cochrane Database of Systematic Reviews, institute for Scientific Information (ISI) “Web of Science,” and Medline. The areas of focus were historical facts, patho-anatomy and patho-physiological correlates of ccTGA, management options and challenges in management in Nigeria.
Results and conclusion: Congenitally corrected transposition of the great artery is a complex cardiac anomaly with a complex anatomy and presents with constellations of features ranging from heart failure, heart block to deaths. The management of ccTGA is a huge challenge in a developing world in general and Nigeria in particular. This is worsened by the harsh economic situation, lack of political will, the current COVID-19 pandemic and lack of state- of-the-art facility and human resources. The cases in Nigeria are been underreported and so are the mortality caused by this cardiac anomaly.
Complex cardiac anomaly; Paediatric cardiology; Mortality; Congenital Heart Disease; COVID-19
Congenital Heart Disease (CHD) is a structural abnormality of heart and associated vascular anomaly that present at the time of birth [1]. The prevalence of congenital heart disease varies between 4 and 10 per 1000 live births [2-4]. Complex heart disease is a group of associated cardiac anomaly involving lesions that are necessary for maintenance of the patient's life [5]. They are often associated with complex alterations in hemodynamics [1,5]. Having a good knowledge of these hemodynamic changes is important to making management decisions, which involve surgery and postoperative management [6].
This complex heart disease include but not limited to hypoplastic left heart syndrome, single ventricle, mitral atresia, pulmonary atresia with intact ventricular septum, tricuspid atresia, double right ventricular outflow tract, double left ventricular outflow tract, Truncus arteriosus, Teratology of Fallot (TOF) with certain syndrome such as TOF with absent pulmonary valve, TOF with pulmonary atresia, Shone’s complex, transposition of great artery with double inlet left ventricle and congenital corrected transposition of the great artery (ccTGA) [7-9].
In developing countries like Nigeria, congenital complex cardiac anomalies are an important cause of mortality and morbidity. This gets worse if there are extra cardiac malformations owing to complications that are linked with such anomalies, necessitating prompt diagnosis and treatment. The aim of this case presentation is to shed light on understanding the anatomy, physiology and clinical correlates of CCTGA and to highlight the challenges associated in its management in resource limited country.
The Prevalence of complex CHD is about 6/1,000 live births. These anomalies contribute between 26 and 37% of all cardiac abnormalities [10]. Congenital corrected transposition of the great artery occurs rarely in our setting and poses a great challenge in management and diagnosis. Congenital corrected transposition of the great artery (ccTGA) is an uncommon congenital heart defect which is often associated with several cardiac anomalies and defect of the conducting system [10].
There are no studies of this rare abnormality in our locale and even in the sub-Saharan African region. The review is therefore aimed at ascertaining a good understanding of ccTGA and their attendant clinical scenarios. Possible complications, management and outcome of this anomaly were also highlighted.
Materials and methods
Data for this review was ascertained from Google scholar, Institute for Scientific Information (ISI) “Web of Science,” PubMed review, and Medline. The search items used were: congenital corrected transposition of the great artery, children; patho-anatomy and pathophysiology of ccTGA; surgical interventions in ccTGA and challenges in management in Nigeria. Additional manual search for studies on ccTGA was done. This was done by highlighting information on ccTGA using references cited in original papers selected for review. Criteria for selection, in this review, are children and adult with ccTGA, diagnosed with angiography, CXR, ECG and echocardiography or those who had surgical intervention for the anomaly.
Embryology/pathophysiology
During embryological development, looping of the heart tube often result in Atrio Ventricular (AV) discordance, and the aortopulmonary septum fails to rotate 180°, with resultant ventriculoarterial discordance [11]. Desaturated blood returns from the body into the right atrium before passing through the mitral valve into the anatomic left ventricle. Blood then enters the lungs through the pulmonic valve into the main pulmonary artery [11-13]. Pulmonary venous blood returns to the left atrium, enters through the tricuspid valve to the morphological right ventricle and exit to the aorta via the aortic valve. The aorta is positioned to the left and anterior of the pulmonary artery (LTGA) [13-15].
Coronary artery anatomy
The coronary arteries in CCTGA arise from the posterior-facing sinuses of the aortic valve. In children with CCTGA with normal situs solitus, the coronary arteries show a mirror-image distribution [16]. The right-sided coronary artery has the distribution of a morphologic left coronary artery. The main right-sided coronary artery bifurcates into circumflex and anterior descending branches, while the left-sided coronary artery courses through the left AV groove, dividing into infundibular and marginal branches [16].
Studies have shown different pattern of coronary artery anomalies. For instance, McKay, et al. [17] noted a persistent origin of the sinus node artery from the circumflex artery coursing through the medial side of the right atrial wall. Atrial baffle procedure or atriotomy repair will be risky in this group of patients [18]. Furthermore, a main coronary branch seen anterior to the pulmonary trunk was seen in 96% of cadaveric specimen the specimens, and a large anterior descending coronary branch crossing the right ventricular outflow tract was seen in 61% of the specimens. Coronaries crossing the RVOT are crucial when considering the Rastelli procedure [19- 21].
Clinical features
The natural history of children with ccTGA is significantly affected by the severity of associated lesions. Although studies have shown long-term survival with ccTGA, however they are not without morbidities. For instance, Beauchesne, et al. [22] in his study on 44 unoperated patients over 144 months, noted 59% of them presenting with morbidities such as severe atrioventricular valve regurgitation with attendant right ventricular dysfunction. This was also similar to the findings of Presbitero, et al. [23]. In the corollary, Graham, et al. [24] in his series noted that children without associated lesions usually present with a lower risk of reoccurrence of heart failure and ventricular dysfunction when compared with those with associated lesions. They also noted that complications and morbidities associated with ccTGA tended to increase in frequency with advancing age [22-25].
Children with congenitally corrected transposition of the great artery usually present with clinical symptoms at the early phase of their lives [16,26]. The diagnosis could be made with the help electrocardiogram done for other reason or as an incidental finding; besides, the anomaly is usually diagnosed later in childhood or at adolescent age when they present with complete heart block [26].
They present commonly with bradycardia with a high-degree AV heart block; a single loud second heart sound, arising from the anteriorly placed aortic valve, heart murmur due to associated ventricular septal defect, tricuspid regurgitation with or without pulmonary stenosis, cyanosis; rhythm disorders and heart failure [27-30].
There could also be situs abnormalities such as Atrial situs, which occurs in in 85%-90% of patients. In addition, shunt defects such as Ventricular septal defect, which is the most common associated cardiac malformation, occurring in 60%-70% of cases [31,32].
The ventricular septal defect is usually sub-pulmonary. Nevertheless, Sub-arterial ventricular septal defects, roofed by the semilunar valves, have been described in Asian patients. The shunt defect is usually a large left-to-right shunt [32].
Children with CCTGA present with conduction abnormalities. The sinus node though normally positioned but the AV conduction tissue is grossly distorted. An aberrated AV node is the functional AV conduction system in many children with ccTGA [33]. It is mainly located juxtaposition to the right atrial appendage just lateral to the pulmonary and mitral valve. This accessory node is often absent but could be hypoplastic or nonfunctional [33].
Complete heart block occurs in 30% of children and could be seen at birth. Sick sinus syndrome, re-entrant AV tachycardia due to an accessory pathway along the tricuspid valve annulus, atrial flutter, and ventricular tachycardia are other conducting abnormalities [34- 36].
Furthermore, children with ccTGA also present with abnormality of the coronary architecture, especially the mirror image location of the anomaly. Dabizzi, et al. noted that over 80% of his series with ccTGA presented with coronary artery-ventricular concordance [16,37]. Left ventricular outflow tract obstruction is noted to occur in 30%-50% of children and is normally associated with VSD.
Freedom, et al. [38] noted that about one third of his patients who had pulmonary outflow tract obstruction and a ventricular septal defect had tricuspid valve deformities. Some children could also present with multiple obstructive lesions, including wedging of the outflow tract by the inverted AV valves, fixed infundibular and valvar pulmonic stenosis and presence of blood cysts attached to the pulmonary valve. Rarely sudden death from severe ventricular fibrillation could occur.
Congenital corrected transposition of the great artery is a complex cardiac anomaly that sometimes presents with abnormal tricuspid valve morphology with incidence rate of 90% in autopsy series, other tricuspid valvar abnormality also includes dysplasia (malformed or imperforate leaflets), (Ebstein-like malformation), straddling and overriding of an inlet ventricular septal defect and straddling or overriding left AV valve, Coarctation of the aorta, Interruption of the aortic arch [31] (Figure 1).
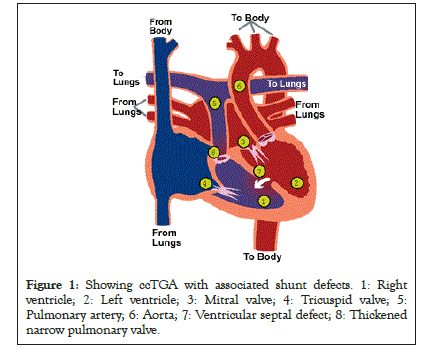
Figure 1: Showing ccTGA with associated shunt defects. 1: Right ventricle; 2: Left ventricle; 3: Mitral valve; 4: Tricuspid valve; 5: Pulmonary artery; 6: Aorta; 7: Ventricular septal defect; 8: Thickened narrow pulmonary valve.
Findings on physical examination depend on the associated anomalies. In patients with large left-to-right shunts, the precordium is hyperactive with displaced apex [28,29]. A quite precordium and cyanosis can be seen in children with pulmonic stenosis. A loud and often palpable single second heart sound is the norm [29].
The murmur of tricuspid regurgitation can be differentiated from that of the typical pansystolic murmur of ventricular septal defect, as the former is maximal at the fourth intercostal space near the sternum and holosystolic [39]. This shows the side-by-side orientation of the ventricles.
Since the pulmonary valve in ccTGA is displaced inferior-posteriorly, the murmur of pulmonary stenosis is usually ascertained at the pulmonary area and loudest on inferior aspect of the aortic area [39].
There are some genetic correlates as aetiologic factors of ccTGA [40]. Children with congenital heart disease have a deletion of chromosome band 22q11. These have been associated with abnormalities of the cono-truncal valves [40].
Investigations
Investigations in ccTGA showed the following; parallel great vessels in chest radiograph. Here, the upper left heart of the heart is formed by the aorta and appears straight, with absent pulmonary artery knob from rightward, posterior displacement of the artery [41,42].
Information on ventricular function, regurgitation of the AV valve, and pulmonary outflow tract could be derived by means of Transesophageal Echocardiography (TEE), especially if the child has undergone an operation [43]. Salient findings on trans-thoracic echocardiography will reveal Tricuspid Valve (TV) being closer to the apex when compared to the mitral valve, the moderator band and thicker trabeculation will be virtualized in the morphological right ventricle which is on the left side of the heart [43]. Besides, a five-chamber view may not be feasible on the left side, however the aorta can be opened in five camber view from right side [43,44]. Furthermore, both the left ventricle and mitral papillary muscles always insert into the free wall while the right ventricle and tricuspid valves insert into one free wall and the septum. The aorta lies left and anterior to the pulmonary artery (LTGA) (Figures 2 and 3).
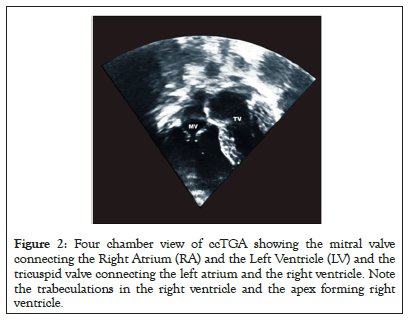
Figure 2: Four chamber view of ccTGA showing the mitral valve connecting the Right Atrium (RA) and the Left Ventricle (LV) and the tricuspid valve connecting the left atrium and the right ventricle. Note the trabeculations in the right ventricle and the apex forming right ventricle.
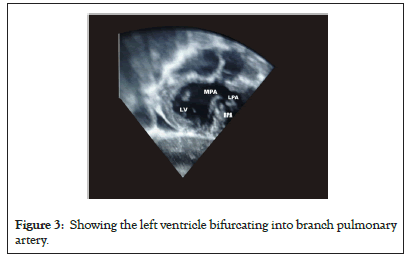
Figure 3: Showing the left ventricle bifurcating into branch pulmonary artery.
Radionuclide angiography provides information on right ventricular function compared with echocardiography [44]. Electrocardiography will show associated cardiac anomalies especially commonly AV block, and atrial arrhythmias, ventricular inversion with attendant reversal of the normal Q-wave pattern in the precordial leads [45]. The ECG in patients with congenitally corrected transposition may therefore be misinterpreted as inferior myocardial infarction. A Holter monitor is used for assessment of AV block and atrial arrhythmia [45].
Cardiac catheterization is not necessarily acceptable in children with ccTGA because it carries a significant risk of inducing transient or complete heart block. However, cardiac catheterization may be necessary in accessing pulmonary vascular resistance in the older patients without any LVOTO [32].
Prenatal diagnosis of ccTGA
Cono-truncal malformations are usually associated with a 22q11.2 deletion and occur in 70% of the heart defects. ccTGA among other types of complex cardiac defect had been implicated [46-50]. Prenatal diagnosis in children with ccTGA is crucial as early diagnosis, identifying medical and surgical correlates before pregnancy in order to improve outcomes, will help prepare the child for early intervention [51]. Fetal echocardiography is a very important tool for guiding management [26].
Prenatal diagnosis of ccTGA can be very tasking. To diagnose ccTGA in utero, double discordance is a sine qua non [26]. Other echocardiographic features that could aid in the diagnosis of corrected transposition. For instance, the location of the right ventricle, identification of the moderator band on the left side of the heart and the apical position of the left-sided tricuspid valvar all tell-tale signs of ccTGA in utero [26].
Familial recurrence of ccTGA is uncommon [26]. Previous studies showed a small number of patients with a large number presenting with situs and looping anomalies including single ventricle, heterotaxia, and shunt defects [26]. A large clinical case series to detect the recurrence of congenital heart defects in families of children with ccTGA over a seven-year period, among 102 patients with ccTGA. The study showed that relatives with congenital heart defects were found in 15% of families with non with ccTGA. Consanguinity was noted in the parents of three probands. Six probands had an unaffected twin-sib [26]. Though ccTGA is not sporadic in families, yet the pattern of inheritance, the recurrence of situs inversus and the presence of consanguinity among parents could suggest autosomal recessive mechanism with similarities with that occurring in some pedigrees with heterotaxia. It is important to note that the recurrence of TGA and ccTGA in the same family suggests a pathogenetic link between these two anatomically different malformations [26].
Situs inversus in ccTGA
Total situs inversus is a rare abnormality in ccTGA [52-55]. The hemodynamics, especially pressure differences in the cardiac chambers and eventual cardiac output could damage the myocardium of children in ccTGA during atrial switch surgery. Children with situs inversus and ccTGA could develop cardiomyopathy and ventricular arrythmias without any surgical intervention [54-56].
Patane, et al. [57] had documented a prevalence of situs inversus in subjects with ccTGA as 17.5%, with 28.5% of them presenting with pulmonary atresia and 9.5% with severe tricuspid regurgitation. About 50% of his subjects also had a good anatomy without any lesion after surgery on those with associated lesions. It is important to note that situs inversus in ccTGA is not a mirror image of situs solitus ccTGA. Children with the former had had a lower risk of tricuspid valve regurgitation than those with the later lesion.
In situ inversus, the morphologic right atrium is located on the morphological left atrium on the right. There is a deficient left-sided infundibular free-wall musculature in the inversus infundibulum but a well-developed right-sided infundibular free- wall musculature. On the other hand, children with situs solitus presents with the morphologic Left Atrium (LA) and Right Atrium (RA) seen on the left and right sides respectively.
Genetic syndromes and extracardiac malformations
Corrected congenital transformation of the great artery like its Transposition of Great Artery (TGA) counterpart is rarely associated with genetic syndromes. A fraction of them may present with Turner, Downs, Williams, Noonan, or Marfan syndromes.
There may be a sporadic occurrence of association of trisomy 8 and 18, with VACTERL and CHARGE syndromesin ccTGA [58,59]. Tuberous sclerosis, has also been documented as extra cardiac association in ccTGA [60]. Besides other extra cardiac anomalies in ccTGA had been reported. This includes DiGeorge/ Velocardiofacial Syndrome and with del22q11 [60].
It is important to note that CCTGA and TGAs may not be seen as pathologic features of cardiac defect of del22q11 syndrome, which is typical in cono-truncal anomalies such as truncus arteriosus, Tetralogy of Fallot, and interrupted aortic arch type B [61]. In fact, it has been documented that children with TGA and ccTGA had only 1% chance of having del22q11 [61-64]. ccTGA and TGAs are common causes of dextrocardia with situs solitus and lateralization defects (Heterotaxy or isomerisms) [60-70]. Other associations seen in ccTGA and TGA are asplenia syndrome (right isomerism), but polysplenia syndrome (left isomerismis rare in ccTGA) [71-79].
Treatment
The neonate with ccTGA and ductal-dependent pulmonary blood flow requires administration of intravenous prostaglandin to maintain patency before definitive surgical intervention [80].
There is paucity of Data and evidence to support positive outcome on the use of ACE inhibitor, beta-blocker, nitrate, hydralazine, aldosterone antagonist as anti-failure regimen in treatment of left ventricular dysfunction and RV failure in the setting of congenitally corrected transposition of the great arteries [81]. It is pertinent to use of beta-blockers with caution as complete heart block may be precipitated in these patients who had established conduction system abnormalities [81].
Intra cardiac repair is recommended when the child is symptomatic with associated shunt lesions with hemodynamic instability [82]. Postoperative complications include complete heart block and worsening tricuspid regurgitation [82-84]. Termignon, et al. [85] in his series noted that 33% of subjects with ccTGA require pace maker post-operation. Two of his series dies with 6 requiring re- operation for tricuspid valve replacement. Importantly, VSD repair could also exacerbate systemic AV valve regurgitation. This valvar regurgitation could be due to septal shift and with attendant distortion of the atrio-ventricular valve annulus [85]. Tricuspid valve repair is not usually feasible, since the valve is dysplastic or even friable. In situations of right ventricular dysfunction and severe tricuspid regurgitation, tricuspid valve replacement could be beneficial [86].
In the presence of pulmonary stenosis and a large ventricular septal defect, double switch procedure could be live saving [86]. However, Chordal mal-attachment of the mitral valve may negate the possibility of double switch maneuver. The Rastelli procedure can be done in children with ccTGA, large ventricular septal defect, and pulmonary outflow obstruction [87]. In this situation, the left ventricle is channelled to the aorta through a baffle via the ventricular septal defect into the right ventricle to the aortic valve. This surgery is then completed with a rastelli [88,89].
Senning or Mustard procedure with additional repair of any ventricular septal defect can be done if the child has a regressed left ventricle or aver small left ventricular mass index [90]. These procedures include baffles at both atria and routing of venous blood to the left ventricle and pulmonary artery, with oxygenated blood being rooted from the pulmonary veins into the right ventricle and to the system [91].
It is worthy of note that the arterial switch operation is recommended as the most appropriate procedure, and should be carried out within 2 weeks of life [91]. Certain conditions must be fulfilled before switch operation. These include; the pressure of the left ventricle must have tolerated near-systemic pressures, the left ventricle should not be regressed with an optimal left ventricular mass index [92].
Pulmonary artery banding can be used to prepare the regressed ventricle prior to a definitive repair. Atrial arrhythmias, pulmonary venous and caval obstruction are late complications of atrial switch operation [93].
Termignon, et al. [85] among his patients noted mortality rate of a classic repair of congenitally corrected transposition of the great arteries and ventricular septal defect to be 16% and the rate of complete heart block as 24% after the repair.
Early pacemaker placement is advocated in the event of complete heart block. This could occur post-operation or in associated defect, such as cardiomegaly, symptomatic bradycardia, or heart failure [85]. It is important to note that this could pose a roller coaster effect as endocardial pacemaker implantation may also trigger an underlying AV valve regurgitation and alteration of the position of the ventricular septum during pacing, with attendant incomplete systolic coaptation of the tricuspid valve [94].
Right ventricular failure can develop as a very late in non-surgical corrected cases [95]. This is usually from coronary perfusion mismatch as the right ventricle is supplied by a single coronary artery. Besides, differences in right and left ventricular fiber orientation may play a role in right ventricular failures [95-98].
Challenges of management of ccTGA in Nigeria
The difficulty in management of ccTGA and other complex cardiac anomaly is usually caused by the lack of facilities for sustainable pediatric cardiac surgical practices in Nigeria. This gross lack of facilities had caused deaths in about 15 million children disease [99].
Even in few parts of the country where these facilities may exist, the cost of surgical intervention is catastrophic and unaffordable, coupled with lack of manpower and skills for double switch operation or even palliative surgery that may be needed in the management of ccTGA. Besides, Nigeria is behooved with tropical diseases such as malaria, tuberculosis, malnutrition and more so the COVD 19 pandemic. As such the country had given priority to these diseases and had treated management of cardiac disease with much triviality [100,101].
In developing country like ours, most surgical intervention for pediatric cardiac lesions is usually handled by cardiac missions and occurs at a later age due to late presentation and diagnosis. For instance, Chinawa, et al. [102] noted a very late presentation for open heart surgery as 4.4 (4.1) years, as against the 6.08 ± 2.80 months seen in Pakistan [103].
Another challenge militating the management of these complex cardiac anomalies in Nigeria is the issue of health financing and insurance. A study has shown that the major source of health financing in children with complex cardiac disease is out-of-pocket payment. This is made up of more than 90% of all types of payment among the caregivers. This calls for an urgent need for health insurance scheme in the country especially in the management of cardiovascular diseases [104].
A high index of suspicion, early diagnosis and quick referral outside are very necessary to enhance the survival of children with ccTGA, especially in children with additional shunt defect [105]. This can be achieved by the provision of affordable human resources, diagnostic and surgical as well as other interventional facilities at each level of care in the country [105,106].
Congenitally corrected transposition of the great artery is a complex cardiac anomaly with a complex anatomy and presents with constellations of features ranging from heart failure, heart block to deaths. The management of ccTGA is a huge challenge in a developing world in general and Nigeria in particular. This is worsened by the harsh economic situation, lack of political will, the current COVID-19 pandemic and lack of state-of-the-art facility and human resources. The cases in Nigeria are been underreported and so are the mortality caused by this cardiac anomaly.
Citation: Chinawa JM (2021) Congenital Corrected Transposition of the Great Artery (ccTGA): A Complex Cardiac Anomaly: Challenges in Management in a Resource Poor Country: A Review Article. J Clin Exp Cardiolog.12:685.
Received: 01-Jun-2021 Accepted: 15-Jun-2021 Published: 22-Jun-2021 , DOI: 10.35248/2155-9880.21.12.685
Copyright: © 2021 Chinawa JM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.