Indexed In
- Open J Gate
- JournalTOCs
- The Global Impact Factor (GIF)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2019) Volume 7, Issue 3
Complementing ADR Data by Utilizing Electronic Health Records:Experience Form a Tertiary Care Hospital in Saudi Arabia
Laila Carolina Abu Esba*Received: 11-Mar-2019 Published: 10-May-2019
Abstract
Objective: Recognizing the limitation of spontaneous adverse drug reaction reporting and the need for better quality of data to monitor the safety of drugs, we explore here the possibilities of complementing the spontaneous ADR reports at an institutional level with data extracted from our electronic health records.
Method: Data on adverse drug reactions documented in patient’s electronic health records was extracted from the hospital’s health information system.
Results: A significant difference in rate and type of adverse drug reactions was observed in comparison to those reported spontaneously by healthcare providers.
Conclusion: implementing a continuous process of complementing hospital based spontaneous adverse drug reaction reporting data with data from patient’s electronic health records can serve as a better tool in improving ADR monitoring.
Keywords
Adverse drug reaction; Electronic healthcare records; Drug
Introduction
An Adverse Drug Reaction (ADR) is defined as “response to a drug which is noxious and unintended and which occurs at doses normally used in man for prophylaxis, diagnosis, or therapy of disease or the modification of physiologic function” [1]. Ever since the thalidomide tragedy and the call for spontaneous ADR reporting as a means to monitor the safety of drugs it has been recognized for its limitations as a methodology that passively detects safety concerns. “Medication without Harm” is the World Health Organization’s (WHO) Third Global Patient Safety Challenge. In which they call for global action “to strengthen the quality of data to monitor harm” [2]. At our institution we acknowledge this limitation and the WHO’s Patient Safety Challenge; we, therefore, explore here the possibilities of complementing the spontaneous ADR reports at an institutional level with data extracted from our Electronic Healthcare Records (EHR).
Underreporting
Underreporting is a worldwide phenomenon, and a significant limitation of interpreting signals from spontaneous ADR reported data. It has been described in many countries, in various healthcare institutions and different healthcare providers. Reasons for underreporting have been linked to lack of time, lack of interest and understanding of its importance, ambiguousness on what to report and others [3-6]. It has been suggested that developing systems to assist healthcare providers with completing ADR reporting within EHRs may increase reporting [7].Over the past couple of years, our team doubled on efforts to improve education and awareness about the importance of reporting, through campaigns and in-service education, and even incentives to top reporting staff and units. Despite these efforts, there was an almost negligible improvement in reporting. As a team, we decided to explore data extracted from our Health Information System (HIS). The WHO previously described methods in complementing spontaneous ADR reporting systems, through Cohort Event Monitoring (CEM) and Targeted Spontaneous Reporting (TSR) utilizing HIS data. These methods can improve detection of drug safety concerns especially with newly added drugs to the market; however these methods are drug specific, adverse effect specific, more cumbersome and do not provide a broad overview of ADRs occurring at an institution [8].
The Ministry of National Guard Health Affairs (MNGHA)
It is a tertiary healthcare system established in 1983 to provide medical care to the National Guard’s soldiers and their dependents in three different regions in Saudi Arabia. MNGHA is a leader in healthcare services in the region [3] with facilities in the Central, Eastern and Western regions of Saudi Arabia. Each region has its own designated ADR team; the ADR team in the Central region is responsible for monitoring and analyzing ADRs reported at King Abdulaziz Medical City the main hospital that has a bed capacity of 1500 beds and for its specialized children’s hospital (King Abdullah Specialized Children Hospital) with a bed capacity of 600.
About our ADR team
At our institution, the clinical pharmacy division leads the ADR program, it is, however, a multidisciplinary team and includes members of the following disciplines: clinical pharmacists, medication safety officer, nursing, physician, and a specialist in bioequivalence and counterfeit drugs.
Workflow
Any MNGHA healthcare provider can spontaneously report ADRs; using the internal electronic Safety Reporting System (SRS). Access to the SRS is through the hospital’s intranet portal. Further, every ADR report undergoes a full evaluation by our ADR team together with the input of the clinical pharmacist covering the unit where the ADR incident took place.
The evaluation process includes:
• Assessing the causality of the ADR utilizing the Naranjo algorithm [9].
• Classifying the event as preventable or not.
• Assigning a level of severity of the ADR utilizing the Hartwig’s severity assessment scale [10].
• A recommendation on whether the ADR should be documented in the patient’s EHR under a section called (ALERT), if not documented already.
• Note: this documentation will result in a system alert should another prescriber order the same drug in the future for the patient.
• Finally, the evaluation includes a section where the clinical pharmacist may enter any comment they have related to the incident.
The ADR team retains the original ADR report together with the clinical pharmacist’s evaluation, and recommend any final actions that need to be taken for an individual ADR case.
Quarterly reporting
The ADR team in addition to constant monitoring for actions required to prevent harm in individual ADR cases reported, compile ADRs, monitor for specific trends on a quarterly base, and make further recommendations to the hospital’s medication safety committee and the pharmacy & therapeutics committee. All ADRs are further sent to the Saudi Food and Drug Authority’s (Saudi-FDA) pharmacovigilance center, contributing locally and internationally to the overall knowledge about the safety of drugs used worldwide [11].
Our Hospital Information System (HIS)
Our in-house built HIS went live in Riyadh region on January 22, 2016. The system is a fully integrated healthcare electronic system that executes all the following functions: Electronic Health Record (EHR), Computerized Physician's Order Entry (CPOE), pharmacy, laboratory and radiology services, special clinic examination, patient services, billing, and social services. Within this system, the medication use process has a closed loop, where documentation of all functions related to direct patient care is stored in one system.
A unique aspect of the system related to ADRs is that there is a specific entry screen for every patient; called the (ALERT) screen (Figures 1 and 2). Under this icon within the HIS, a patient’s encountered ADR can be documented. This documentation allows the system to trigger a warning should any other prescriber order the drug for the patient at any point in the future.
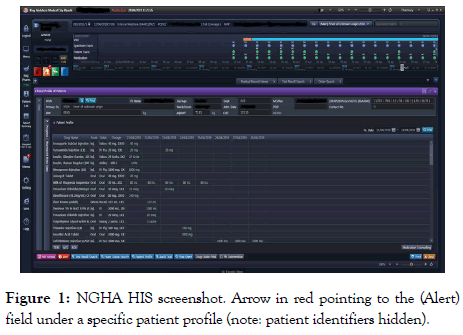
Figure 1. NGHA HIS screenshot. Arrow in red pointing to the (Alert) field under a specific patient profile (note: patient identifiers hidden).
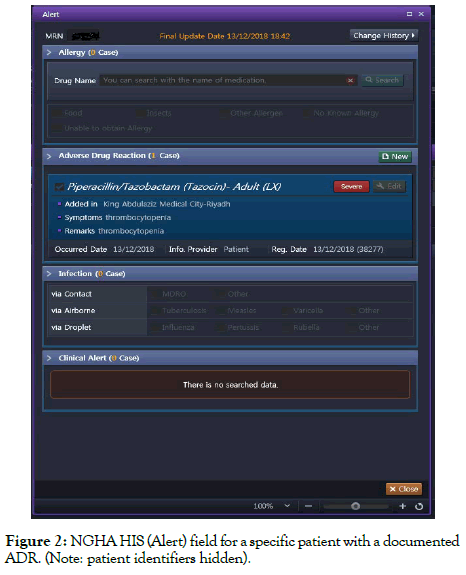
Figure 2. NGHA HIS (Alert) field for a specific patient with a documented ADR. (Note: patient identifiers hidden).
Method
This was a retrospective observational study based on the data collected from ADRs reported in the SRS and the ADRs extracted from the EHRs for all patients at MNGHA-Riyadh. We collected data entered under the ALERT section within the EHR's between the period of January 2016 until December 2018; and compared it to the number and trends of ADRs spontaneously reported through the SRS during the same period.
Setting
The study included ADRs that occurred in NGHA patients admitted or followed up in the outpatient setting, in both NGHA facilities in Riyadh region: King Abdulaziz Medical City and King Abdullah Specialized Children Hospital. This study was approved by the local Ethics Committee of King Abdulaziz Medical City (IRB approval RC19/066/R, approved in May 2019).
Data Collection
Our ADR team requested access to the data entered under the (ALERT) icon within the EHRs for all patients at MNGHA-Riyadh, the data requested included:
• Patient’s medical record number.
• Patient’s demographics (age, date of birth, gender).
• Name of the drug.
• The ADR encountered (i.e., signs or symptoms).
• Severity (classified as mild, moderate, or severs).
• Date of ADR occurrence, and remarks (which is a free text space the healthcare provider can add any relative information regarding the ADR).
We were granted access after a formal approval process, and access was provided in the form of an internal link that is continuously updated and fed by the data entered within the HIS. (Figure 3) shows how the data extracted from EHRs is presented on a dashboard provided by the HIS team. All ADRs reported through the SRS were assessed by a clinical pharmacist; the causality assessment was done utilizing the Naranjo algorithm. As this is an initial exploratory study of the data extracted from the EHR and due to the large number of ADRs from this source, no individual case assessment for each ADR extracted from the EHRs was conducted. Therefore all ADRs whether with a certain, probable, or possible causality assessment were included.
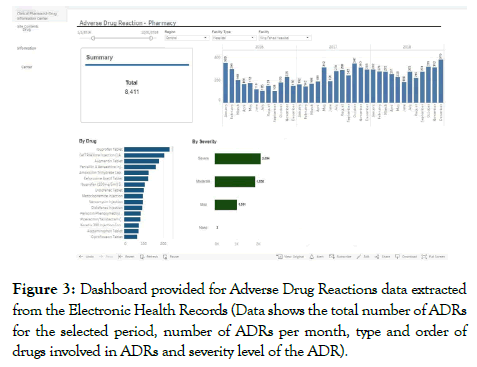
Figure 3. Dashboard provided for Adverse Drug Reactions data extracted from the Electronic Health Records (Data shows the total number of ADRs for the selected period, number of ADRs per month, type and order of drugs involved in ADRs and severity level of the ADR).
Findings
ADR Rates and Frequencies
Total number of ADRs reported through SRS was 207, 205, and 266 for years 2016, 2017, and 2018 respectively. ADRs extracted from the EHRs were 2,215, 2,922, and 3,273 for years 2016, 2017, and 2018 respectively. Compared to what is reported through the SRS the number of ADRs from the data extracted from the EHRs indicates that only 10% of the ADRs documented were reported (Figure 4).
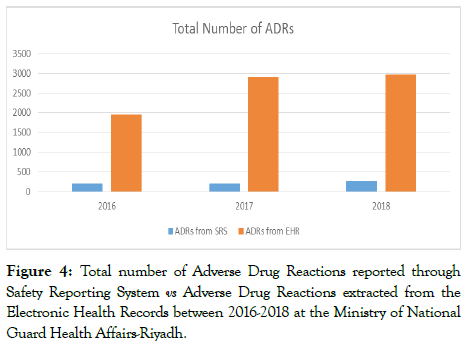
Figure 4. Total number of Adverse Drug Reactions reported through Safety Reporting System vs Adverse Drug Reactions extracted from the Electronic Health Records between 2016-2018 at the Ministry of National Guard Health Affairs-Riyadh.
Top Drugs Causing ADRs
A significant finding on examining the data since Jan 2016 until December 2018 was that the drug that was reported to have the highest number of ADRs reported through our SRS was not the highest drug shown by the data extracted from the EHRs. Our top reported ADRs through the SRS was related to ceftriaxone allergic type reactions, while in the data extracted from the EHRs ceftriaxone came second after Ibuprofen; which was unforeseen as we had almost zero ADR reports related to Ibuprofen through the SRS, and it was never among our top reported ADRs between 2016-2018.
Monitoring Trends
As a result of the low number of ADR reports received through our SRS, it was impossible to build a trend for each medication against its utilization as a tool to monitor for signals. The added data from the EHRs on ADRs allowed us to explore the methodologies of signal detection on an institutional level. This is important for detecting local practice issues, drug quality surveillance and rapid response to drug safety concerns. An example of one drug’s ADRs being plotted to visualize its historical trend (Figure 5).
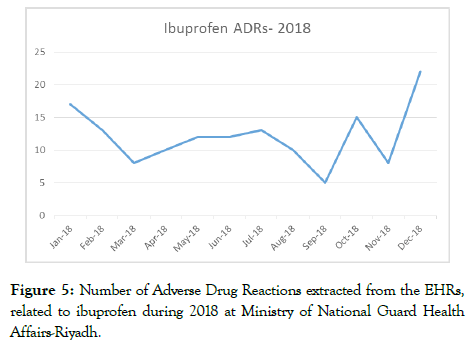
Figure 5. Number of Adverse Drug Reactions extracted from the EHRs, related to ibuprofen during 2018 at Ministry of National Guard Health Affairs-Riyadh.
Discussion
Compared to the high incidence of ADRs reported in studies such as Pirmohamed et al. [12] and Lazarou et al. [13]. The spontaneously reported ADRs did not correlate with the expected incidence of ADRs in our large tertiary care hospital. Compared to what is reported through the SRS the number of ADRs from the data extracted from the EHRs indicates that only 10% of the ADRs documented were reported. On the other hand, not all ADRs are either documented by the healthcare provider within the EHR; demonstrating the very small proportion of ADRs that are actually reported. A couple of large scale projects have taken place in recent years as an initiative to integrate observational data from EHRs in assisting in signal detection such as the Pharmacoepidemiological Research on Outcomes of Therapeutics by a European Consortium (PROTECT) and the EU-ADR project. Trifiro[14] describe their findings from utilizing large EHR data from the EU-ADR project as useful for detecting signals related to ADRs of higher incidents but may be less powered to detect rare ADRs [15,16]. These projects have mainly focused on a limited set of adverse events and are large country based projects, and therefore up to our knowledge, are different from our attempt to utilize such EHR data at a hospital level to complement the spontaneous reporting system with a more generalizable effort in detecting local safety issues. The difficulty of mining through EHR has been approached with some suggested methods [17], nevertheless we had the advantage of our self-tailored HIS in which we could easily extract ADR data. In our EHR there is a specified ADR entry screen for every patient, the system also regularly alerts physicians on every patient admission/encounter to update this precaution screen with patient’s ADR/Allergy. It was substantial to recognize the discrepancy between what was reported through SRS and the data provided from the EHR on ADRs; opportunities to improve patient safety may have been missed by relying on spontaneous ADR reporting alone. Utilizing the information from EHR will further assist us in mitigating harm and identifying new safety concerns. Over the past couple of years as a response to ceftriaxone being our top drug linked to ADRs we took the following actions:
• Reported the concern to the Saudi-FDA and requested they perform a quality test for impurities of selected batches.
• Reported the concern to the manufacturer of the brand we had in stock.
• We developed guidance for healthcare providers on how to manage cross-reactivity in patients allergic to cephalosporin.
Unfortunately, no actions were taken in regards to ibuprofen as it was never reported through the SRS despite it being the top drug listed in the ADRs extracted from the EHR; therefore opportunities to improve patient safety may have been missed. Reasons for underreporting ADRs have been described in many studies, and have included uncertainty about the causality, lack of awareness on how and where to report, workload, and fear of liability [18]. Efforts and interventions on how to improve reporting have been suggested; however, this seems to be an inevitable limitation that could never be adequately addressed by campaigns or incentives. Techniques in signal detection are difficult to apply with a limited number of spontaneous reports received; by utilizing, the large number of ADRs provided from the EHRs detecting safety signals may be more attainable. As seen from the example in Figure 4 for ADRs related to one drug (Ibuprofen) establishing a historical trend in relation to the proportion of the total number of prescription at the institution, can serve as a tool for signal detection by utilizing methodologies such as ‘observed vs expected’ or ‘continuous disproportionality’ analysis.
Recommendations
• For those in the phase of building their HIS, allow a designated screen for ADR entry, in order to retrieve the data efficiently and as needed.
• Utilize the data from the EHR to complement the data from the spontaneous ADR reporting system; this would provide a more accurate indication of safety concerns.
• Educate staff on the importance of accurate ADR documentation in the designated section within the EHR.
• Continue to support efforts in improving ADR spontaneous reporting.
Limitations
ADRs documented in the HIS are still subject to humanistic errors of documentation, and therefore a process should be in place not only to improve awareness about the importance of accurate ADR documentation but also a process for validating the data extracted and ensuring it represents an accurate reflection of the ADRs occurring. A study showed a high discrepancy in ADR documentation in a newly implemented HER system and recommended implementing processes that improve completeness and continuity of ADR documentation [19]. Therefore, our next attempt would be to establish a systematic method for our team to identify inaccurate ADRs documented and exclude them from our analyses and interpretation of the data. It is important to note that ADRs with high uncertainty about causality, either due to lack of previous experiences, an unexpected effect of the drug, a long time to develop, or complicated by other comorbidities will likely not be documented in patient’s EHRs and are likely to be missed. Therefore, spontaneous ADR reporting may remain as a better tool for detecting rare ADRs.
Conclusions
At our institution, we have implemented this continuous process of complementing our spontaneous ADR reporting data with ADR data from our EHRs. We are enthusiastic that we will get more signals in the near future and be able to detect drug safety concerns faster and more importantly with better accuracy.
REFERENCES
- Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician's guide to terminology, documentation, and reporting. Ann Intern Med. 2004;140:795-801.
- Donaldson LJ, Kelley ET, Dhingra-Kumar N, Kieny MP, Sheikh A. Medication without harm: who's third global patient safety challenge. Lancet. 2017;389:1680-1681.
- Hazell L, Shakir SA. Under-reporting of adverse drug reactions. Drug Saf. 2006;29:385-96.
- Gahr M, Eller J, Connemann BJ, Schönfeldt-Lecuona C. Underreporting of adverse drug reactions: Results from a survey among physicians. Eur Psychiat. 2017;41:69.
- Haider N, Mazhar F. Factors associated with underreporting of adverse drug reactions by nurses: A narrative literature review. Saudi J Health Sci. 2017;6:71.
- Varallo FR, Guimarães SD, Abjaude SA, Mastroianni PD. Causes for the underreporting of adverse drug events by health professionals: a systematic review. Rev Esc Enferm Usp. 2014;48:739-747.
- Ribeiro-Vaz I, Silva AM, Santos CC, Cruz-Correia R. How to promote adverse drug reaction reports using information systems–a systematic review and meta-analysis. BMC Med Inform Decis Mak. 2016;16:27.
- Pal SN, Duncombe C, Falzon D, Olsson S. WHO strategy for collecting safety data in public health programmes: Complementing spontaneous reporting systems. Drug saf. 2013;36:75-81.
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. American Journal of Health-System Pharmacy. 1992;49:2229-2232.
- The National Pharmacovigilance and Drug Safety Center. (n.d.). Retrieved from https://www.sfda.gov.sa/en/drug/about/sector_departments/national_pharmacovigilance_center/sector_departments_national/Pages/Vigilance_invo.aspx
- Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. Bmj. 2004;329:15-19.
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. Jama. 1998;279:1200-1205.
- Trifiro G. What is the additional value of electronic medical records for drug safety signal detection? The experience of eu-adr project. Clin Ther. 2013;35:130-131.
- Trifirò G, Patadia V, Schuemie MJ, Coloma PM, Gini R, et al. EU-ADR healthcare database network vs. spontaneous reporting system database: preliminary comparison of signal detection. Stud Health Technol Inform. 2011;166:25-30.
- Pharmacoepidemiological Research on Outcome of Therapeutics by a European Consortium PROTECT Home. (n.d.). Retrieved from http://www.imi-protect.eu/
- Haerian K, Varn D, Vaidya S, Ena L, Chase HS, et al. Detection of pharmacovigilance‐related adverse events using electronic health records and automated methods. Clin Pharmacol Ther. 2012;92:228-234.
- Vallano A, Cereza G, Pedròs C, Agustí A, Danés I, et al. Obstacles and solutions for spontaneous reporting of adverse drug reactions in the hospital. Brit J Clin Pharmaco. 2005;60:653-658.
- Hui C, Vaillancourt R, Bair L, Wong E, King JW. Accuracy of adverse drug reaction documentation upon implementation of an ambulatory electronic health record system. Drugs-real World Outcomes. 2016;3:231-238.
Citation: Esba LCA (2019). Complementing ADR Data by Utilizing Electronic Health Records: Experience Form a Tertiary Care Hospital in Saudi Arabia. J Pharmacovigil 7:276
Copyright: © 2019 Esba LCA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


