PMC/PubMed Indexed Articles
Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 12, Issue 4
Comparative Quality Evaluation of Different Brands of Clopidogrel Tablet Available in Bangladesh
MD Naimur Rehman*Received: 03-May-2021 Published: 24-May-2021, DOI: 10.35248/2157-2518.21.12.365
Abstract
This study aims to assess the quality parameters of Clopidogrel tablets from different manufacturers available in the Bangladeshi market. Clopidogrel is a potent antiplatelet and antithrombotic drug. Clopidogrel has been approved and marketed globally with the primary indication being to reduce atherosclerotic events in patients with several comorbid conditions due to stroke, myocardial infarction and cardiovascular disease. The aim and the objective of our present study are to evaluate the quality parameters of some marketed Clopidogrel tablet and to compare the parameters among them. To assess the quality, three different marketed Clopidogrel 75 mg tablet are selected and invitro dissolution test, potency, disintegration time are carried out. Other general quality parameters of these tablets like weight variation, hardness, friability are also determined according to established protocols. All the brands comply the requirements of United State Pharmacopoeia as they show acceptable weight variation range. Friability of all brands is less than 1%. No significant differences are found in disintegration time as they disintegrate within 15 minutes. In case of dissolution profile all brands show better dissolution time as they release more than 75% of drug in 45 minutes. The hardness of one brand is within the range 40-60N. The limitation of the potency must be within 95- 105%. All three brands meet this specification. This study suggests that most commercially available Clopidogrel tablets in Bangladesh maintain the quality and comply with the USP specifications which are essential for better therapeutic activity of this antiplatelet drug.
Keywords
Quality Evaluation; Clopidogrel Tablet; Pharmacopoeia; antiplatelet drug; diphosphate.
Introduction
Drug Profile
Clopidogrel, an antiplatelet agent structurally and pharmacologically similar to ticlopidine, is used to inhibit blood clots in a variety of conditions such as peripheral vascular disease, coronary artery disease, and cerebrovascular disease. Clopidogrel is an inhibitor of ADP-induced platelet aggregation acting by direct inhibition of adenosine diphosphate (ADP) binding to its receptor and of the subsequent ADP-mediated activation of the glycoprotein GPIIb/IIIa complex. Chemically it is methyl (+)-(S)-α(2- chlorophenyl)-6,7-dihydrothieno[3,2- c]pyridine-5(4H)-acetate. The empirical formula of Clopidogrel bisulfate is C16H16ClNO2S• H2SO4 and its molecular weight is 419.9.
The empirical formula of Clopidogrel (free base) is C16H16ClNO2S and its molecular weight is 321.82. The melting point is 158°C and water solubility is 50.78 mg/L at solid state (Figure 1).
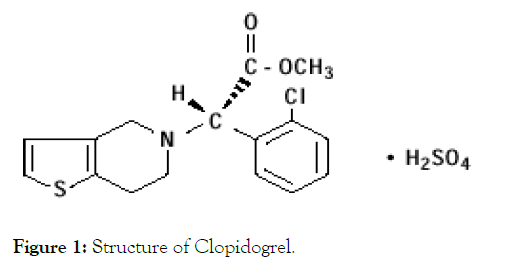
Figure 1: Structure of Clopidogrel.
Clopidogrel bisulfate is a white to off-white powder. It is practically insoluble in water at neutral pH but freely soluble at pH 1.
It also dissolves freely in methanol, dissolves sparingly in methylene chloride, and is practically insoluble in ethyl ether. It has a specific optical rotation of about +56° [1].
Synthesis
The first synthetic route for (S)-(+)-Clopidogrel bisulfate 1* H2SO4 as shown in Scheme 1 started with the condensation of o-chloro benzaldehyde 2 with 4, 5, 6, 7-tetrahydrothieno [3, 2- c] pyridine 3 by employing Strecker synthesis that afforded cyano intermediate 4 [2].
Subsequently, cyano intermediate 4 converted to amide 5. Reaction of amide 5 in methanol and acidic reagent afforded racemic Clopidogrel, which was resolved using Lcamphorsulfonic acid to afford (S)-(+)-Clopidogrel 1 (Figure 2).
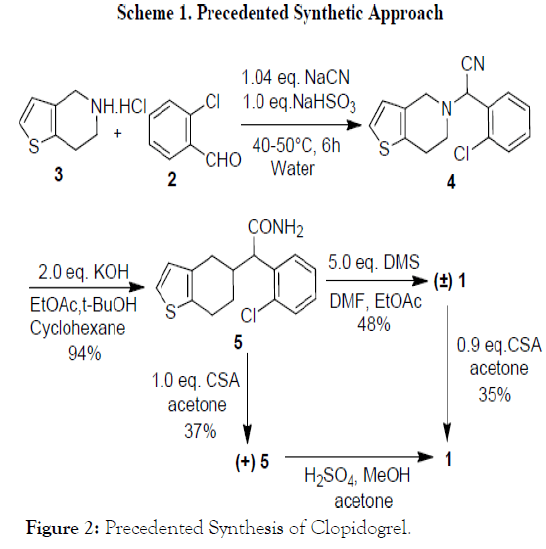
Figure 2: Precedented Synthesis of Clopidogrel.
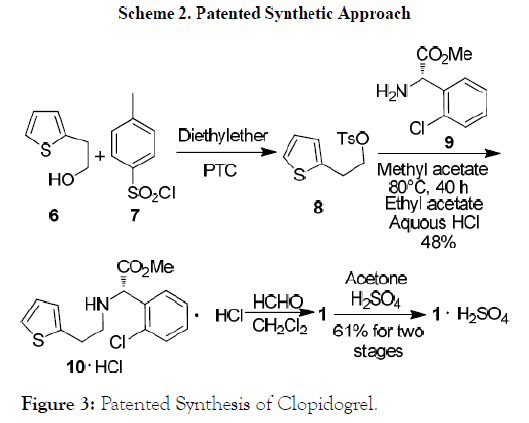
Figure 3: Patented Synthesis of Clopidogrel.
The second synthetic route for preparation of (S)-(+)-Clopidogrel 1 as shown in Scheme 2 commences with tosylation of thiophene-2-ethanol 6 with p-toluenesulfonyl chloride (p- TSA) 7 in the presence of phase transfer catalyst affording 2-(2-Thienyl) ethyl tosylate 8, which upon reaction with (+)-α-amino-2-chloro phenyl acetic acid methyl ester 9 afforded substituted intermediate 10·HCl.
Finally, this intermediate undergoes reaction with formaldehyde followed by electrophilic cyclization and salt formation to obtain bisulfate salt of 1* H2SO4.
Clinical pharmacology
Mechanism of Action and Pharmacodynamic Properties: Clopidogrel is a prodrug, one of whose metabolites is an inhibitor of platelet aggregation. A variety of drugs that inhibit platelet function have been shown to decrease morbid events in people with established cardiovascular atherosclerotic disease as evidenced by stroke or transient ischemic attacks, myocardial infarction, unstable angina or the need for vascular bypass or angioplasty. This indicates that platelets participate in the initiation and/or evolution of these events and that inhibiting platelet function can reduce the event rate [3].
Clopidogrel must be metabolized by CYP450 enzymes to produce the active metabolite that inhibits platelet aggregation. The active metabolite of Clopidogrel selectively inhibits the binding of adenosine diphosphate (ADP) to its platelet P2Y12 receptor and the subsequent ADP-mediated activation of the glycoprotein GPIIb/IIIa complex, thereby inhibiting platelet aggregation. This action is irreversible. Consequently, platelets exposed to Clopidogrel’s active metabolite are affected for the remainder of their lifespan (about 7 to 10 days). Platelet aggregation induced by agonists other than ADP is also inhibited by blocking the amplification of platelet activation by released ADP (Figure 4).
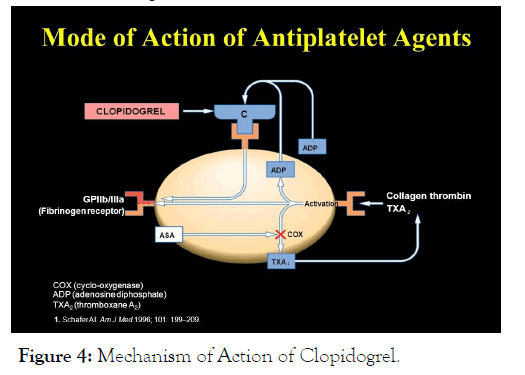
Figure 4: Mechanism of Action of Clopidogrel.
Because the active metabolite is formed by CYP450 enzymes, some of which are polymorphic or subject to inhibition by other drugs, not all patients will have adequate platelet inhibition. Dose dependent inhibition of platelet aggregation can be seen 2 hours after single oral doses of Clopidogrel. Repeated doses of 75 mg Clopidogrel per day inhibit ADP-induced platelet aggregation on the first day, and inhibition reaches steady state between Day 3 and Day 7. At steady state, the average inhibition level observed with a dose of 75 mg Clopidogrel per day was between 40% and 60%. Platelet aggregation and bleeding time gradually return to baseline values after treatment is discontinued, generally in about 5 days [4].
Pharmacokinetics data bioavailability
50% Protein binding: 94–98% Metabolism: liver Onset of action: 2 hours Biological half-life: 7–8 hours (inactive metabolite) Duration of action: 5days Excretion: 50% kidney; 46% biliary [5].
Pharmacokinetics
Absorption: After single and repeated oral doses of 75 mg per day, Clopidogrel is rapidly absorbed. Mean peak plasma levels of unchanged Clopidogrel (approximately 2.2-2.5 ng/mL after a single 75 mg oral dose) occurred approximately 45 minutes after dosing. Absorption is at least 50%, based on urinary excretion of Clopidogrel metabolites [6].
Effect of Food: The effect of food on the bioavailability of the parent compound or active metabolite is currently not known.
Distribution: Clopidogrel and the main circulating inactive metabolite bind reversibly in vitro to human plasma proteins (98% and 94%, respectively). The binding is nonsaturable in vitro up to a concentration of 100 mcg/mL.
Metabolism: Clopidogrel is extensively metabolized by the liver. In vitro and in vivo, Clopidogrel is metabolized according to two main metabolic pathways: one mediated by esterase and leading to hydrolysis into its inactive carboxylic acid derivative (85% of circulating metabolites), and one mediated by multiple cytochromes P450. Cytochromes first oxidize Clopidogrel to a 2- oxo-Clopidogrel intermediate metabolite. Subsequent metabolism of the 2oxo-Clopidogrel intermediate metabolite results in formation of the active metabolite, a thiol derivative of Clopidogrel. In vitro, this metabolic pathway is mediated by CYP3A4, CYP2C19, CYP1A2 and CYP2B6. The active thiol metabolite, which has been isolated in vitro, binds rapidly and irreversibly to platelet receptors, thus inhibiting platelet aggregation [7].
Elimination: Following an oral dose of 14C-labeled Clopidogrel in humans, approximately 50% of total radioactivity was excreted in urine and approximately 46% in feces over the 5 days post-dosing. After a single, oral dose of 75 mg, Clopidogrel has a half-life of approximately 6 hours. The elimination half-life of the inactive acid metabolite was 8 hours after single and repeated administration. Covalent binding to platelets accounted for 2% of radiolabel with a half-life of 11 days. In plasma and urine, the glucuronide of the carboxylic acid derivative is also observed. Indications and Usage Clopidogrel (Clopidogrel bisulfate) is indicated for the reduction of atherothrombotic events as follows: Recent MI, Recent Stroke or Established Peripheral Arterial Disease For patients with a history of recent myocardial infarction (MI), recent stroke, or established peripheral arterial disease, Clopidogrel has been shown to reduce the rate of a combined endpoint of new ischemic stroke (fatal or not), new MI (fatal or not), and other vascular death [8].
Acute Coronary Syndrome–For patients with non-ST-segment elevation acute coronary syndrome (unstable angina/nonQ-wave MI) including patients who are to be managed medically and those who are to be managed with percutaneous coronary intervention (with or without stent) or CABG, Clopidogrel has been shown to decrease the rate of a combined endpoint of cardiovascular death, MI, or stroke as well as the rate of a combined endpoint of cardiovascular death, MI, stroke, or refractory ischemia. For patients with ST-segment elevation acute myocardial infarction, Clopidogrel has been shown to reduce the rate of death from any cause and the rate of a combined endpoint of death, re-infarction or stroke. This benefit is not known to pertain to patients who receive primary angioplasty [9].
Adverse Reactions: Serious adverse drug reactions associated with Clopidogrel therapy include:
Thrombotic thrombocytopenic purpura: (incidence: four per million patients treated) Hemorrhage –the annual incidence of hemorrhage may be increased by the coadministration of aspirin
Quality analysis
Weight Variation Test: According to the USP weight variation test is run by weighting 20 tablets individually calculating the average weights and comparing the individual tablet weights to the average. The value of weight variation test is expressed in percentage. The following formula is used: Weight Variation= {(Iw - Aw)/Aw} x 100%
Where, Iw=Individual weight of tablet and Aw=Average weight of tablet.
As per USP the tablet complies with the test if not more than 2 of the individual masses deviate from the average mass by more than the percentage deviation as shown in Table 1 and none deviates by more than twice that percentage (Table 1).
| Average Weight (mg) | % deviation |
|---|---|
| 130 mg or less | 10.0% |
| More than 130 mg and less than 324 mg | 7.5% |
| More than 324 mg | 5.0% |
Table 1: USP limits for weight variation test for uncoated tablets.
Diameter Test: Uniformity of the diameter of tablet follows the pharmacopoeial standard. All of the percentage average deviation value must not exceed±5% for tablets with diameter less than 12.5 and±3% for diameter of 12.5 mm or more. The following formula is used: Deviation={[|Initial diameter Average diameter|] / Average diameter} x 100%
Thickness Test: The thickness of a tablet is the only dimensional variable related to the process. Thickness of individual tablets may be measured by a micrometer. Other techniques involve placing 5 or 10 tablets in a holding tray, where their total thickness may be measured by a sliding caliper scale. Tablet thickness should be controlled within a±5% variation of a standard. Thickness must be controlled to facilitate packaging. It is expressed in mm [10].
Disintegration Test: Disintegration test is performed to find out that within how much time the tablet disintegrates. Disintegration test is very important for all coated and uncoated tablet because the dissolution rate of drug depends on the disintegration time, which ultimately affect the rate of absorption of drug (Table 2).
| Categories of Tablets | Disintegration Time (min) |
|---|---|
| Uncoated tablets | 15 |
| Coated tablets% | 60 |
| Effervescent tablets | 5 |
| Soluble tablets | 3 |
| Dispersible tablets | 3 |
| Orodispersible tablets | 3 |
| Gastro-resistant tablets | 3 |
Table 2: BP limits for disintegration times of tablets
Hardness Test: For this test one of the earliest testers was Ketan tablet hardness tester, which is a type of the Monsanto hardness tester to evaluate tablet hardness tester. The tester consists of a barrel containing a compressible spring held between two plungers. The lower plunger is placed in contact with the tablet and zero reading is taken. The upper plunger is then forced against a spring by turning a threaded bolt until the tablet fractures. As the spring is compressed, a pointer rides along a gauge in the barrel to indicate the force. The force of fracture is recorded in kilogram. Ten tablets are crushed and measure their hardness and the allowable range is between 4-6 kg (40-60 N) unless otherwise specified.
Friability Test: Friability of a tablet can determine in laboratory by Roche friabilator. For this test twenty tablets are weighed and placed in the friabilator and then operated at 25 rpm for 4 minutes. The tablets are then dedusted and weighed. The difference in the two weights is used to calculate friability and the value of friability is expressed in percentage. It is determined by the following formula: Friability={(Iw - Fw)/Iw} x 100% Where, Iw=Total Initial weight of tablets and Fw=Total Final weight of tablets As stated by USP if conventional compressed.
Tablets that loss less than 0.5 % to 1 % (after 100 revolutions) of their weight are generally considered acceptable.
Potency Test: This test is done for determining the toxic and therapeutic effect of the drug. The potency of the tablet should comply with the specification because very highly potent drug may give toxic effect and very less potent drug may give subtherapeutic effect. %Potency=Concentration (mg/ml) x Dilution Factor x Total Volume (ml) x Average Weight (mg) x 100 Sample Taken (mg) x Strength (mg).
Dissolution Test: The transfer of a drug from its solid dosage form into the solution of GI fluid as dissolved form is called dissolution. The slowest step in a series of kinetic process of drug is called the rate-limiting step. For drug that have very poor aqueous solubility, the rate at which the drug dissolves is often the rate-limiting step and thus exerts a rate-limiting effect on drug bioavailability. Therefore, it is necessary to test the dissolution of drug (Table 3).
| Stage | No. of tablet tested | Acceptance criteria |
|---|---|---|
| S1 | 6 | Each unit is not less than Q + 5 %. |
| S2 | 6 | Average of 12 units (S1 + S2) is equal to or greater than Q, and no unit is less than Q – 15 %. |
| S3 | 12 | Average of 24 units (S1 + S2 + S3) is equal to or greater than Q, not more than 2 units are less than Q – 15 %, and no unit is less than Q – 25 %. |
Table 3: BP, USP, PhEur, JP and PhInt acceptance criteria for dissolution test of tablet.
Materials and Methods
Study Outline: Quality parameter tests of three different brands of clopidogrel were carried out in laboratory using various apparatus and reagents.
Sample Collection: Three different brands of 75 mg clopidogrel tablets (20 tablets of each brand) were obtained from local pharmacy in Dhaka. The batch number, manufacturing license number, date of manufacture and expiry dates were checked. The products were checked for its shelf life and no samples were collected having shelf life less than 2 years (Table 4).
| Name | Function |
|---|---|
| Standard Clopidogrel | Reference standard powder |
| Phosphate buffer, pH 7.4 | For media preparation |
Table 4: Chemicals used in the experiments.
Methods weight variation test
To carry out the weight variation test at first 20 clopidogrel tablets from each of the 3 brands were weighed individually. Then the average weight was calculated and the average weight is compared to the individual weight of the tablet to find out the variation. By using the following formula weight variation is calculated.
Average weight = (X1+X2+X3+………+Xn)/20
Weight Variation = (Individual weight-average weight) x 100/ average weight.
For a tablet to pass the test not more than two tablets should lie out the specified percentage and if no tablets differs by more than two times the percentage limit (Table 5).
| Average Weight (mg) | % deviation |
|---|---|
| 130 mg or less | 10.0% |
| More than 130 mg and less than 324 mg | 7.5% |
| More than 324 mg | 5.0% |
Table 5: According to USP the Percentage Limits.
Measurement of thickness and diameter.
To perform this test, 20 clopidogrel tablets were taken. Then each tablet was placed in digital slide caliper horizontally and vertically to measure the thickness and diameter of the tablet respectively (Figure 5).

Figure 5: Slide Caliper.
Hardness test
To perform this test, 3 Clopidogrel tablets were taken individually and placed vertically in an automatic hardness tester and set up perfectly. Then the machine was pressed to check the hardness of the tablet at which point the tablet broke. The hardness of the tablets was measured using tablet hardness tester. It is expressed in Newton. Hardness of tablet should be optimum because–Too hard tablet may not disintegrate Too soft tablet may break during handling, packaging and transporting Factors that Affects Hardness are Compression of the tablet and compressive force Amount of binder (More binder, more hardness) Method of granulation in preparing the tablet (Figure 6).

Figure 6: Hardness Tester.
Specification: Normal coated tablet hardness ranges from 4-8 kg (1 kg=10 Newton)
Friability Test
To perform this test 7 clopidogrel tablets were weighed and placed at the Roche friabilator where they were exposed to rolling and repeated shocks as they fall 6 inches in each turn within the apparatus.
After 4 minutes of this treatment or 100 revolutions the tablets are weighed and the weight compared with initial weight.
The loss due to abrasion is a measure of tablet friability. The value is expressed in percentage. Friability below 1% is considered acceptable. % Friability = [(W1 –W2) /W1] × 100
Here, W1=Weight before the test and W2= Weight after the test (Figure 7).

Figure 7: Friabilator.
Disintegration Test
According to USP, the disintegration apparatus consists of 6 glass tubes with a 10 number mesh at the bottom, each tube is 3 inch long. This arrangement of 6 tubes is placed in a medium simulated to the disintegration environment. A thermostatic arrangement for heating the liquid and maintaining the temperature at 37°±2° .This system is made to move up and down through a distance of 5 to 6 cm at a frequency of 28 to 32 cycles per minute. To perform this test, 3 clopidogrel tablets from each brand were employed in Phosphate buffer, pH 7.4 media at 37°C using a Tablet Disintegration Tester. The disintegration time was taken as the time when no particle remained on the basket of the system (Figure 8).

Figure 8: Disintegration Tester.

Figure 9: UV Spectrophotometer
Preparation of standard curve of clopidogrel
To begin with 10 mg of clopidogrel was weighed and taken in a 100 ml volumetric flask. Then a few ml of Phosphate Buffer pH 7.4 was added to dissolve the powder. After the powder completely dissolved, Phosphate Buffer pH 7.4 was added upto the 100 ml mark and a solution of 100 mcg/ml was formed. 80 ml from that solution was taken in a 100 ml volumetric flask and volume was adjusted up to 100 ml with Phosphate Buffer pH 7.4 to make solution of 80 mcg/ml. Then 1 ml solution from previous solution was taken and 9 ml Phosphate Buffer pH 7.4 was added to make a solution of 8 mcg/ml. Similarly 2 ml, 3 ml, 4 ml and up to10 ml stock solution was withdrawn and volume was adjusted up to 10ml to make concentrations 16-80 mcg/ml. Then absorbance was taken of the respective solutions by UV spectrophotometer at 222 nm against blank and the absorbance was plotted against the concentration to obtain the standard curve using Microsoft Excel.
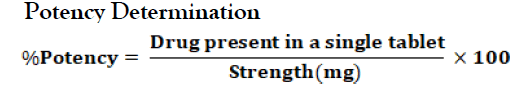
At first, four tablets of each brand were weighed together and then the average weight was determined. Those four tablets were crushed properly and equivalent weight was measured by using weight balance and then dissolved into 100ml of Phosphate Buffer pH 7.4 and was shaken for good mixing. After that the preparation was filtered and the absorbance was taken. As the taken absorbance was greater than 1 so it was diluted. 80 ml filtrate was taken and volume adjusted up to 100 ml with Phosphate Buffer pH 7.4. Then the absorbance was taken at 222 nm by UV spectrophotometer against a blank and potency was calculated using the calibration curve of reference standard clopidogrel with the following formula.
Dissolution Test
Dissolution test of the tablets were performed using USP dissolution apparatus II (paddle) in 900 ml Phosphate Buffer pH 7.4 as dissolution media, at 50 rpm and 37±0.5ºC temperature. Just when the temperature reached 37ºC, one tablet was dropped into the vessel and the paddle was allowed to rotate. Test sample (10ml) was withdrawn at particular time (at 5, 15, 30, 45, and 60) interval and replaced with fresh dissolution media of same volume and maintained at the same temperature. Collected samples were filtered and analyzed by UV Spectrophotometer at 222 nm against blank. Release rate were calculated as percent of drug release with using the calibration curve (Figure 10).

Figure 10: Dissolution Tester.
Results and Discussion
Weight variation of clopidogrel tablets (75 mg)
Weight of 20 Clopidogrel tablets of each of the three brands was determined to study their weight uniformity along with their weight variations from tablet to tablet. The results obtained should be within limits allowed by USP specifications (generally ±10% for tablets weighing 130 mg or less, ± 7.5% for tablet weighing more than 130 mg to 324 mg and ± 5% for tablet weighing more than 324 mg).The table below shows the results obtained (Table 6).
| Brand Name | Average Weight (mg) | Maximu m Weight (mg) | Minimu m Weight (mg) | Maximu m % Deviation | Maximu m % Deviation |
|---|---|---|---|---|---|
| A | 267.45 | 276 | 261 | 3.20% | -2.41% |
| B | 270.85 | 283 | 268 | 4.49% | -1.05% |
| C | 219.75 | 226 | 217 | 2.84% | -1.25% |
Table 6: Average Weight and Maximum, Minimum % Deviation of Clopidogrel Brands.
The table above shows the results acquired from weight variation test. According to the outcome, brand B had the maximum average weight (270.85 mg) followed by brand A (267.45 mg) and among the three brands; brand C has the lowest average weight (219.75 mg). In terms of deviations from average weight, brand B showed maximum deviation 4.49% and brand A showed minimum deviation -2.41%. Average weight of three different brands of Clopidogrel tablets is shown in the following bar diagram (Figure 11).
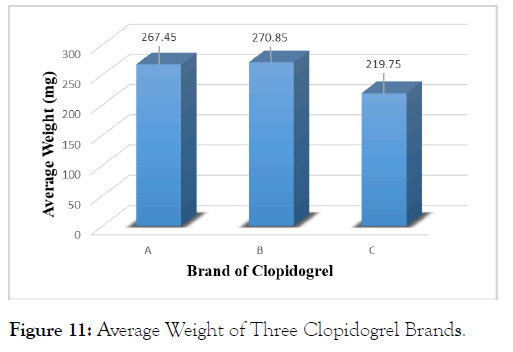
Figure 11: Average Weight of Three Clopidogrel Brands.
Thickness of clopidogrel tablet (75 mg)
The weight of a compressed tablet is dependent on three factors and thickness is of one them. Along with the determination of weight variation, the thickness of the 20 tablets from each brand was measured by using electronic digital caliper and their average thickness was also calculated (Table 7).
| Brand Name | Average | Highest (mg) | Lowest | Maximum % Deviation | Maximum % Deviation |
|---|---|---|---|---|---|
Thickness (mm) |
Thickness (mm) | Thickness (mm) | |||
| A | 4.226 | 4.29 | 4.16 | 1.51% | -1.56% |
| B | 3.7035 | 3.78 | 3.67 | 2.07% | -0.90% |
| C | 4.167 | 4.21 | 4.07 | 1.03% | -2.33% |
Table 7: Average Thickness of Different Clopidogrel Brands.
According to the values obtained from the thickness test, brand A exhibited the maximum average thickness (4.2260mm) among the three brands and brand B showed the lowest average thickness of (3.7035mm). And brand B showed the highest maximum % deviation in thickness 2.07% whereas brand C showed the highest minimum % deviation in thickness -2.33%. Average thickness of three different brands under investigation is shown in the following diagram.
Diameter of Clopidogrel Tablet (75 Mg)
The diameter of a tablet is as important as the thickness and measured in the same way using slide calipers. The table below shows the result obtained from the measurement of diameter of each tablet of different brands (Table 8).
| BrandName | Average Diameter | Highest Diameter | Lowest Diameter | Maximum % Deviation | Minimum%Deviation |
|---|---|---|---|---|---|
| (mm) | (mm) | (mm) | |||
| A | 9.32 | 9.37 | 9.27 | 0.54% | -0.54% |
| B | 10.16 | 10.18 | 10.13 | 0.20% | -0.30% |
| C | 8.26 | 8.28 | 8.22 | 0.24% | -0.48% |
Table 8: Diameter of Different Clopidogrel Brands.
Hardness of clopidogrel tablet (75 mg)
Tablet hardness serves both as a criterion to guide product development and as a quality- control specification. Thus the hardness of three tablets from each brand was measured to determine the breaking of the tablets. The results were obtained in Newton unit and are given (Table 9).
| Brand Name | Tab-1 | Tab-2 | Tab-3 | Average |
|---|---|---|---|---|
| A | 39 | 37 | 39 | 38.34 |
| B | 45 | 50 | 51 | 48.67 |
| C | 32 | 31 | 35 | 32.67 |
Table 9: Hardness (N) of Different Clopidogrel Brands.
Interpreting the table above, brand B showed the maximum average hardness among the three brands and brand C showed the minimum average hardness.
Friability of clopidogrel tablet (75 mg)
Friability of a tablet is also an important quality parameter and it is interrelated to hardness. Friability is presented in percentage. 7 tablets of each brand were weighed and placed in the apparatus and exposed to rolling and repeated shocks as they fall 6 inches in each turn within the apparatus. After four minutes, the tablets were weighed and the weight was compared with the initial weight. The loss due to abrasion was a measure of the tablet friability using friabilator (Table 10).
| Brand | W1 (mg) | W2 (mg) | % Friability |
|---|---|---|---|
| A | 1882 | 1880 | 0.106 |
| B | 1914 | 1913 | 0.052 |
| C | 1551 | 1550 | 0.064 |
Table 10: Friability of Different Clopidogrel Brands.
The % friability of brand B (0.052%) was least among the three brands and brand A (0.106%) showed the highest % of friability. Percentage friability of 3 different brands of clopidogrel tablet is shown in the following diagram.
Disintegration time of clopidogrel tablet (75 mg)
The test was conducted on the three different brands of Clopidogrel tablet. According to USP, disintegration time for clopidogrel tablet should be not less than 75% at 45 minutes (2700 sec).
According to the data, the average time of three tablets required to disintegrate in the media was highest was for brand B among the three brands.
Standard Curve of Clopidogrel
To determine the potency of Clopidogrel a standard curve was required. At first 10 mg of reference standard clopidogrel was taken and dissolved in 100 ml Phosphate Buffer pH
7.4 solution. From there 80 ml of the solution was taken and adjusted up to 100 ml by adding 20 ml of phosphate buffer. From that solution by appropriate serial dilutions, standard solutions were prepared with concentrations ranging from 8 mcg/ml to 80 mcg/ml. Then absorbance data for the corresponding concentrations were collected from UV spectrometer.
Absorbance’s were plotted against concentration and standard curve (y=0.012x+0.001, R²=0.99) was obtained with the help of Microsoft Excel.
Determination of potency of clopidogrel tablets (75 mg)
Potency of each brand of Clopidogrel tablet was determined by using the equation obtained (y = 0.012x + 0.001, R2 = 0.99) from the standard curve of reference standard Clopidogrel. The calculated amount of potency of three different brands.
Discussion
The quality control parameters of the three brands of clopidogrel were tested in this study. The average weight, average thickness and average diameter of the tablets from all the three brands are within the USP limit specifications. These assure pharmaceutical equivalence of drug products After the weight variation, thickness and diameter test, the hardness test were carried out. As per the results, brand B showed the highest average hardness followed by brand A and finally brand C showed the lowest average hardness among the three brands. The results from the friability test showed highest % friability for brand A and lowest % friability for brand B. The highest time to disintegrate was taken by brand B tablets and lowest time was taken by brand A and brand C showed inter-mediate results. Brand A showed the maximum % release of drug in 60 minutes, brand C showed the lowest percent of drug release. Since all the brands showed more than 75% release of drugs at 45 minutes, it can concluded that clopidogrel is an immediate release dosage form. According USP specifications, the potency of drug should be in the range 90%-110%. The potency test results showed that all the three brands contained the right amount of active ingredient and the % potency was within the range with brand B having the highest percentage potency and brand A with lowest potency. Since this test is used to determine the amount of active ingredient present and whether it complies with pharmacopeia specifications so correlation with other quality parameters are not stated.
Conclusion
Multi brands for a specific generic drug are available in the market. And the efficacy of one brand might be better than the other irrespective of being produced by a leading or small level company. In-vitro laboratory tests of quality control parameters play a significant role in the comparison of different brands. This study illustrates that all three brands of Clopidogrel showed acceptable results for the chemical and physical tests including assay, dissolution, hardness, thickness, diameter, disintegration, and dissolution and % potency but one brand being better than the others in terms of % potency and dissolution rate.
REFERENCES
- Abernethy DR. Pharmacokinetics and pharmacodynamics ofamlodipine. J Health Sci. 1992;80(1):31–36.
- Danafar H, Hamidi M. Pharmacokinetics and bioequivalence study of amlodipine and atorvastatin in healthy male volunteer by LC-MS. Pharm Sci. 2015;21(1):167-174.
- Feroz M, Razvi N, Ghayas S, Anjum F, Ghazal L. Assessment of pharmaceutical quality control and equivalence of various brands of amlodipine besylate (5mg) tablets available in the Pakistani market under biowaiver conditions. Int J Pharm Sci. 2014;6(1): 909-913.
- Danafar H, Hamidi M. Pharmacokinetics and bioequivalence study of amlodipine and atorvastatin in healthy male volunteer by LC-MS. Pharm Sci. 2015;21(1):167-174.
- Goyal J, Khan ZY, Upadhaya P, Goyal B, Jain S. Comparative study of high dose mono-therapy of amlodipine or telmisartan, and their low dose combination in mild to moderate hypertension. J Clin Diagn Res. 2014;8(6):8-11.
- Haque S, Brishti NR, Noor M, Das S, Shahriar M. Comparative invitro equivalence evaluation of some desloratadine generic tablets marketed in Bangladesh. Int J Applied Res. 2017;3(2):288-293.
- Madivada LR, Anumala RR, Gilla G. An efficient and large scale synthesis of Clopidogrel: Antiplatelet drug. Der Pharma Chemica. 2012;4(1):479-488.
- Nandhini S. Essential hypertension: a review article. J Pharm Sci Res. 2014;6(9):305-307.
- Reddy PV, Roy SD, Vassavi G, Ram NS, Yadev M. Formulation and evaluation of oral disintegrating tablets of amlodipine by direct compression method using sublimating agents. Int J Phar Res. 2014;4(1):56-63.
- Stangier J, Schmid J, Turck D. Absorption, metabolism, and excretion of intravenously and orally administered telmisartan and amlodipine in healthy volunteers. J Clin Pharmacol. 2000;40(1): 1312–1322.
Citation: Rehman MDN (2021) Comparative Quality Evaluation of Different Brands of Clopidogrel Tablet Available in Bangladesh. J Carcinog Mutagen. 12:365.
Copyright: © 2021 Rehman MDN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


