Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review - (2021) Volume 0, Issue 0
Bright Line Eating®: A Two-year Follow-up Evaluation of a Commercial Telehealth Weight Loss Program within an Abstinence-Based Food Addiction Framework
Susan Peirce Thompson1, Andrew Kurt Thaw2*, Mark G Goetting3 and Win Guan32Department of Psychology and Neuroscience, Director of Pre-Health at Millsaps College, USA
3Departments of Pediatric and Adolescent Medicine and Medicine, Western Michigan University School of Medicine, USA
Received: 05-Mar-2021 Published: 25-May-2021
Abstract
Objective: The current study evaluates two-year weight outcomes for participants of the Bright Line Eating: Boot Camp program (BLE:BC), a weight management program that teaches participants to abstain from sugar and flour within a food addiction framework.
Methods: Data come from participants in the BLE:BC Follow-up Research Program. Participants were invited to complete monthly follow-up surveys. First, we examined primary outcomes of percent weight loss (%WL) and change in body mass index (BMI) for participants who completed both the BLE:BC program and at least one followup survey. Next, we examined %WL and BMI change only among participants who completed all surveys.
Results: An independent samples analysis showed that, at the time of each follow-up survey (6, 12, 18, and 24 months), participants reported clinically significant weight loss (>5%WL) from baseline. Weight loss at each followup was significantly greater than at the end of the BLE:BC program (>7.9%WL). Among participants who completed all surveys (n=238), weight loss was higher among participants enrolled in the Bright Lifers continuity program at 12, 18, and 24 months. At the 24-month follow-up, participants enrolled in the Bright Lifers program experienced an average 15.3%WL and a 5.0 reduction in BMI.
Conclusion: A rigorous evaluation of the efficacy of commercial weight loss programs remains imperative. As research on the construct of food addiction in humans’ increases, evaluating treatment approaches becomes increasingly important. Although the generalizability of the current study is limited due to selection bias and sample homogeneity, this study contributes significant findings to the literature showing sustained, long-term weight loss among participants in the BLE:BC program.
Keywords
Obesity; Body weight; Overweight; Weight loss; Body mass index; Weight management; Food addiction; Abstinence; Telehealth; Bright line eating
Introduction
According to the 2017-18 National Health and Nutrition Examination Survey, more than two in five American adults (42.4%) suffer from obesity (body mass index ≥ 30). Because obesity is associated with increased mortality risk [1] and is comorbid with such diseases as type 2 diabetes, hypertension, and heart disease [2], the high obesity rate in the United States represents a significant public health concern. This is especially important during the novel coronavirus (COVID-19) pandemic, given the association between obesity and greater risk of hospitalization, mechanical ventilation, and mortality [3].
Research shows that behavioral interventions can produce weight loss of 8-10 percent of initial weight (%WL) within 6 months [4]. However, those who lose weight often regain much of it within a year [5]. In-person interventions have been shown to be more effective than web-based interventions for weight loss, but the latter are significantly more effective in slowing weight regain, because of the ongoing support that these programs can provide [5]. As a result, web-based approaches have become popular components within clinical interventions, and as a result, commercial weight loss programs. This is significant given that commercial weight loss programs represent a $3.7 billion industry that is expected to experience an annual 6% growth rate [6].
However, as shown in two systematic reviews [7,8] of intervention designs and outcomes of commercial weight loss programs, studies evaluating weight loss success produce inconsistent results given the variation in program design, intensity, and the inclusion of various nutrition and/or physical activity components. Moreover, these studies focus largely on short-term weight loss, creating a need for studies of long-term outcomes [9-11]. To address this gap, the present study examines two-year weight loss results of individuals participating in the Bright-Line Eating: Boot Camp (BLE:BC) program, an 8-week commercial weight loss intervention based on a food addiction framework.
Literature Review
This study builds on a prior evaluation of the BLE:BC program that showed significant weight loss results among participants immediately post-intervention [12].
Bright line eating
Bright Line Eating is grounded in a food addiction framework. The scientific literature offers little research on the efficacy of non-profit 12-Step programs, such as Overeaters Anonymous, that advocate an abstinence-based food plan [13]. Bright Line Eating is the first widespread commercial program grounded in a food addiction framework. Participants are educated about the neuroscientific evidence that overeating can be an addiction and about the overlap between drug addiction and processed food addiction [14,15]. As a weight loss, weight management, and food addiction treatment approach, the BLE program employs an abstinence model, similar to quitting smoking, which promotes strict adherence to four “Bright Lines”--behavioral boundaries that participants aim not to cross under any circumstances. The first Bright Line involves abstinence from added sugar or sweeteners of any kind (no sugar, honey, corn syrup, molasses, artificial sweeteners, etc.) and the second involves abstinence from flour of any kind (white flour, whole-grain flour, almond flour, coconut flour, etc.). The plan encourages and builds into the food plan consumption of fructose found in whole foods, such as fresh fruit. More information on the structure of the BLE:BC program can be found elsewhere [12].
Participants
The current study evaluates weight loss outcomes among individuals who completed a Bright Line Eating Boot Camp (BLE:BC) and enrolled in the BLE:BC Follow-up Research Program. All BLE:BC participants were invited to complete webbased pre- and post-intervention surveys. Following completion of the BLE:BC program, participants were also invited to join the BLE:BC Follow-up Research Program, which consists of monthly follow-up surveys to measure the process, outcome, and impact of the BLE:BC program. Additionally, those who completed the BLE:BC program could enroll in a continuity program called Bright Lifers, which provides ongoing support through an online community, online special interest groups, group coaching calls, and continued education. All participants provided informed consent and received a detailed protocol regarding data use, security, and management. Upon consultation with the IRB, we confirmed the research did/does not require review because it is not considered a clinical investigation as defined by the FDA and the dataset utilized did not include identifiable private information Criteria for inclusion in the current study were that individuals must have completed a BLE:BC prior to June 1, 2017, completed the pre- and post-intervention surveys, and enrolled in the BLE:BC Follow-up Research Program.
The current study uses data from the pre- and post-intervention surveys and the 6-month, 12-month, 18-month, and 24-month follow-up surveys. Participants were excluded if data were missing or if they represented outliers (z-score > ±3) for the primary outcome measures. As shown in Figure 1, among those who completed the BLE:BC pre- and post-intervention surveys and subsequently enrolled in the BLE:BC Follow-up Research Program (n=905), 75.8% (686) completed the 6-month follow-up, 56.4% (510) completed the 12-month follow-up, 42.4% (384) completed the 18-month follow-up, and 32.5% (294) completed the 24-month follow-up. Figure 2 shows the step-wise exclusion of participants from the pre-intervention survey, leading to the restricted sample— individuals who completed all seven surveys (n=238).
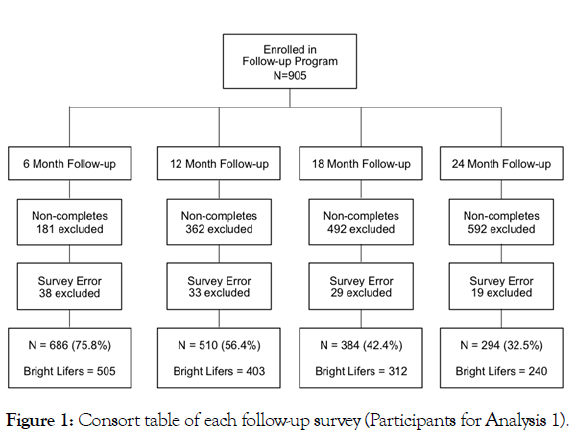
Figure 1: Consort table of each follow-up survey (Participants for Analysis 1).
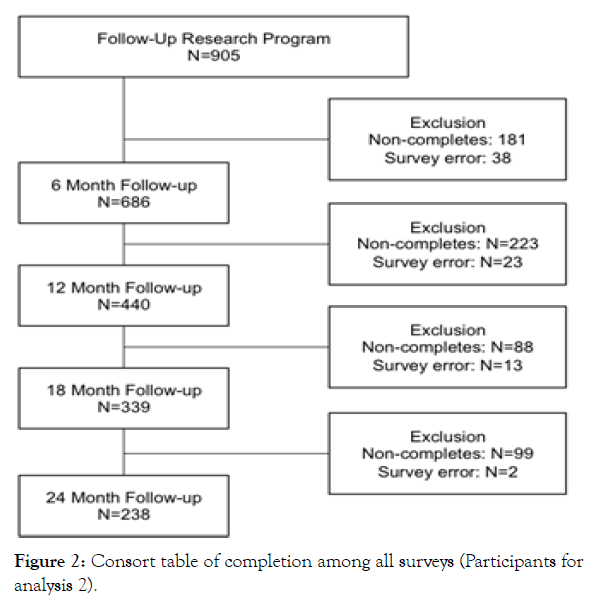
Figure 2: Consort table of completion among all surveys (Participants for analysis 2).
Measures
This study examines two primary outcome measures: weight loss and BMI change. We collected self-reported anthropometric data in each survey. We calculated percent weight loss (%WL) as the percentage of pre-intervention weight that participants lost. To calculate body mass index, we used the standard formula (kg/ m2). We stratified the primary outcomes by baseline weight status (normal, overweight, type 1 obesity, type 2 obesity, and type 3 obesity). Additionally, we examined differences in weight loss and BMI change across follow-ups by whether the participant enrolled in the Bright Lifers continuity program. We collected baseline demographic information from the pre-intervention survey.
Analytic strategy
Using STATA SE 12.0, we conducted the analysis in two parts. First, we examined %WL and BMI change among participants who completed the pre-intervention survey and at least one followup survey (sample sizes shown in Figure 1). That meant, then, that participants who did not complete all follow-up surveys were included in that analysis. Because that created different samples of the study population at different time points, trend analyses were not possible. The second analysis accounted for that fact by restricting the sample to only participants who participated in the post-intervention survey and all follow-up surveys; we refer to this below as the restricted sample (Figure 2). This second analysis examined the trajectory of %WL and BMI change across all time points and variation by whether participants enrolled in the Bright Lifers continuity program.
Results
Baseline characteristics of the total and restricted samples appear in Table 1. The majority of the total and restricted sample members are female (94.3% and 91.7%, respectively) with an average age of 53.9 in the total sample and 52.2 in the restricted sample. Additionally, the majority of the participants in both samples identify as white (91.8% and 95.5%, respectively). The average baseline BMI in both samples (33.1 and 31.5, respectively) falls above the threshold for type 1 obesity.
| Variables | Total Sample (n=905) | Restricted Sample (n=238) | ||
|---|---|---|---|---|
| Mean (%) | SD | Mean (%) | SD | |
| Sex (Female) | 94.3% | -- | 91.7% | -- |
| Age | 53.9 | 10.6 | 52.2 | 13.2 |
| Race (White) | 91.8% | -- | 95.5% | -- |
| Height (m) | 1.5 | .09 | 1.6 | .08 |
| Weight (kg) | 87.4 | 20.6 | 86.7 | 18.4 |
| Body Mass Index | 33.1 | 7.6 | 31.5 | 6.5 |
Table 1: Descriptive statistics (Pre-intervention).
Figure 3 presents the percent weight loss, stratified by baseline weight status, for each follow-up survey. On average, participants experienced 7.9%WL (SD=4.1) in the post-intervention survey following completion of the 8-week BLE:BC program. Among those who completed each particular follow-up research survey, participants experienced a 15.2%WL (SD=8.0) for the 6-month follow-up survey sample, 16.8%WL (SD=10.8) for the 12-month follow-up survey sample, 15.3%WL (SD=11.4) for the 18-month follow-up survey sample, and 14.3%WL (SD=10.8) for the 24-month follow-up survey sample. For each survey sample, participants whose weight fell within the normal BMI range at baseline experienced lower %WL than those who were overweight or obese at baseline. For instance, in the 6-month follow-up, participants who fell within normal BMI range at the beginning of the Boot Camp reported 9.0%WL (SD=5.2), on average, compared to an average 16.5%WL (SD=8.1) among participants who began the program with type 3 obesity.
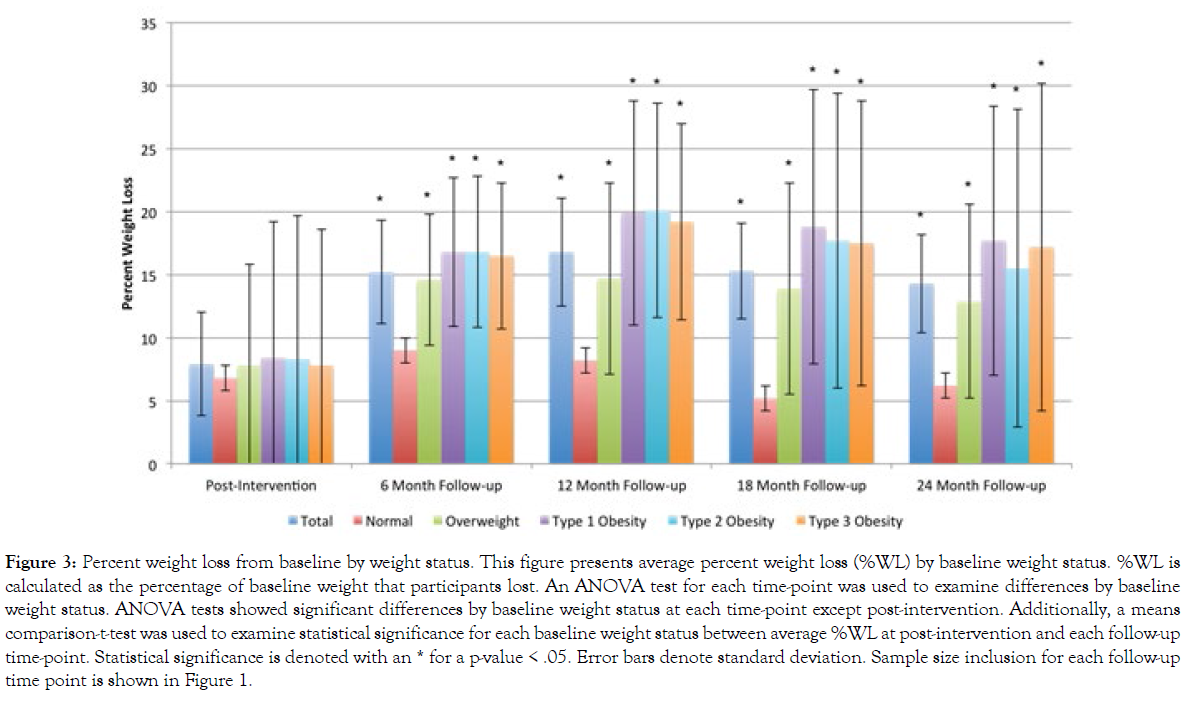
Figure 3: Percent weight loss from baseline by weight status. This figure presents average percent weight loss (%WL) by baseline weight status. %WL is calculated as the percentage of baseline weight that participants lost. An ANOVA test for each time-point was used to examine differences by baseline weight status. ANOVA tests showed significant differences by baseline weight status at each time-point except post-intervention. Additionally, a means comparison-t-test was used to examine statistical significance for each baseline weight status between average %WL at post-intervention and each follow-up time-point. Statistical significance is denoted with an * for a p-value < .05. Error bars denote standard deviation. Sample size inclusion for each follow-up time point is shown in Figure 1.
That difference became even more pronounced in subsequent follow-ups. For instance, participants in the 12-month follow-up survey who reported type 3 obesity at baseline experienced over two times the %WL, on average, of participants whose weight fell within normal BMI range at baseline (19.2%WL and 8.2%WL, respectively). In the 18- and 24-month survey samples, the %WL of those who reported type 3 obesity at baseline stood at nearly three times that of those who began the program within normal BMI range.
Figure 4 shows the percent of BLE:BC participants who began the program with obesity and experienced various clinically significant weight loss at increasing increments. Among participants who were obese at baseline who completed the post-intervention survey (n=1132), 19.2% experienced less than 5%WL, 44.5% experienced 5-10%WL, 33.7% experienced 10-15%WL, and 2.6% achieved more than 20%WL. The distribution is more evenly spread within the 6-month follow-up sample (n=520), with a high percentage of individuals who experienced 20-25%WL and individuals who experienced over 25%WL (16.7% and 22.4%, respectively). In the 12-month, 18-month, and 24-month samples, the percentage of participants who experienced over 25%WL increased significantly (to 37.2%, 35.9%, and 31.6%, respectively). However, the percentage of participants who experienced <5%WL also increased from 6 months (to 12.1%, 13.2%, and 17.1%, respectively).
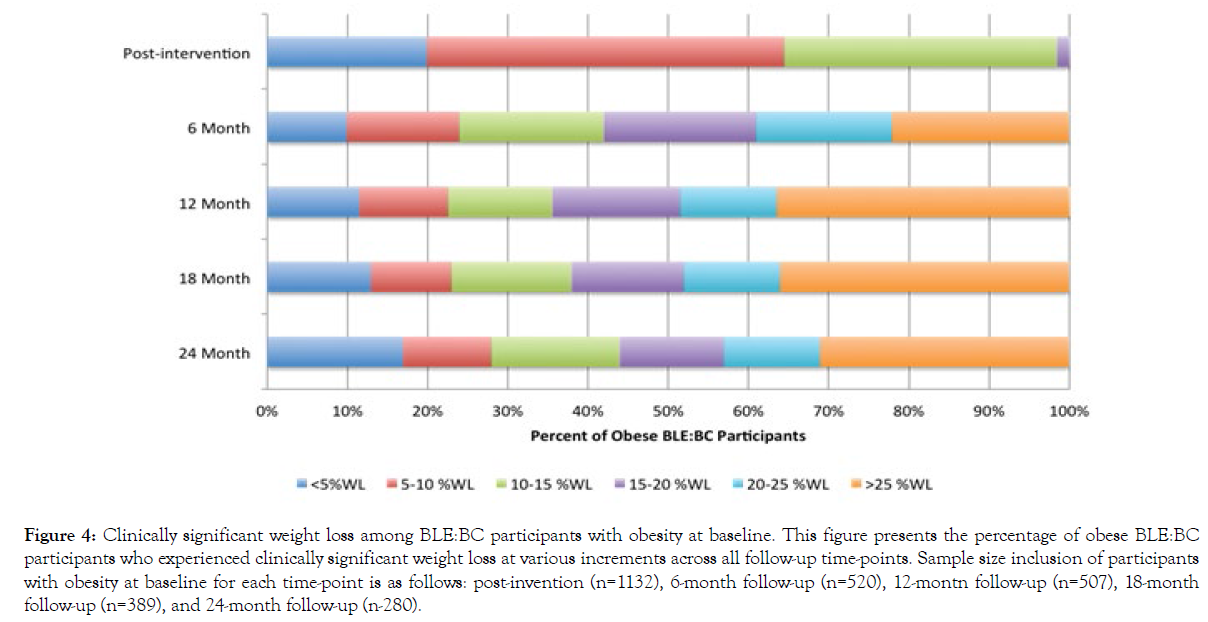
Figure 4: Clinically significant weight loss among BLE:BC participants with obesity at baseline. This figure presents the percentage of obese BLE:BC participants who experienced clinically significant weight loss at various increments across all follow-up time-points. Sample size inclusion of participants with obesity at baseline for each time-point is as follows: post-invention (n=1132), 6-month follow-up (n=520), 12-montn follow-up (n=507), 18-month follow-up (n=389), and 24-month follow-up (n-280).
Figure 5 presents BMI change among participants in each survey sample. On average, participants experienced a BMI decrease of 2.6 (SD=1.4) immediately following completion of the BLE:BC program. Among those who completed each particular follow-up research survey, participants saw a BMI decrease of 5.0 (SD=3.0) in the 6-month follow-up survey sample, a BMI decrease of 5.6 (SD=4.1) in the 12-month follow-up survey sample, a BMI decrease of 5.1 (SD=4.2) in the 18-month follow-up survey sample, and a BMI decrease of 4.7 (SD=4.0) in the 24-month follow-up survey sample. The difference in BMI change between participants who began the program within normal BMI range and participants who reported being overweight or obese at baseline exceeded the differences we found for %WL. For instance, participants in the 24-month follow-up survey who reported type 3 obesity at baseline experienced an average BMI decrease of 7.5 (SD=5.6), compared to an average BMI decrease of 1.4 (SD=1.4) among participants who fell within normal BMI range at baseline.
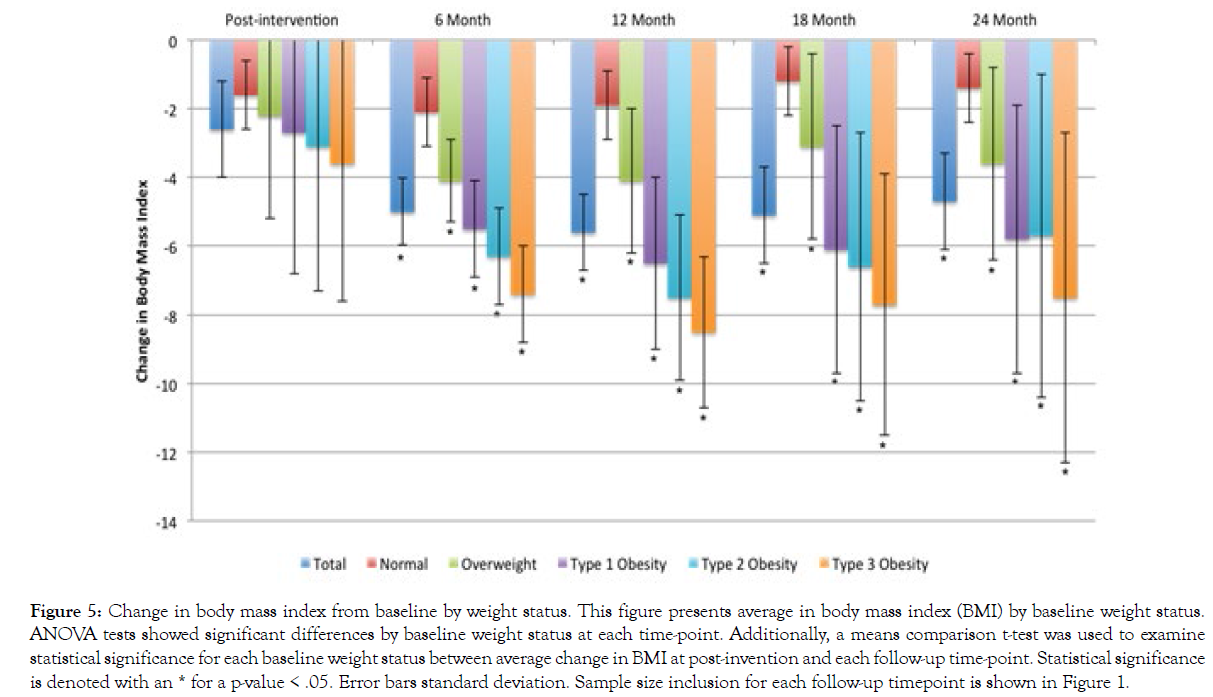
Figure 5: Change in body mass index from baseline by weight status. This figure presents average in body mass index (BMI) by baseline weight status. ANOVA tests showed significant differences by baseline weight status at each time-point. Additionally, a means comparison t-test was used to examine statistical significance for each baseline weight status between average change in BMI at post-invention and each follow-up time-point. Statistical significance is denoted with an * for a p-value < .05. Error bars standard deviation. Sample size inclusion for each follow-up timepoint is shown in Figure 1.
Additionally, we examined whether enrolling in the Bright Lifers continuity program affected participants’ %WL and BMI reduction. Figure 6 presents results for %WL and Figure 7 presents results for BMI change. Using an independent t-test comparison of means, results show that participants who enrolled in the Bright Lifers program experienced greater %WL at all follow-up time-points than participants who did not become Bright Lifers (6-month t(684)=3.58, p<.001; 12-month t(508)=6.08, p<.001; 18-month t(382)=4.33, p<.001; 24-month t(292)=4.49, p<.001). Similarly, participants enrolled in the Bright Lifers continuity program experienced a greater decrease in BMI than those who did not enroll (6-month t(684)=3.37, p<.001; 12-month t(508)=5.34, p<.001; 18-month t(382)=4.09, p<.001; 24-month t(292)=3.98, p<.001).
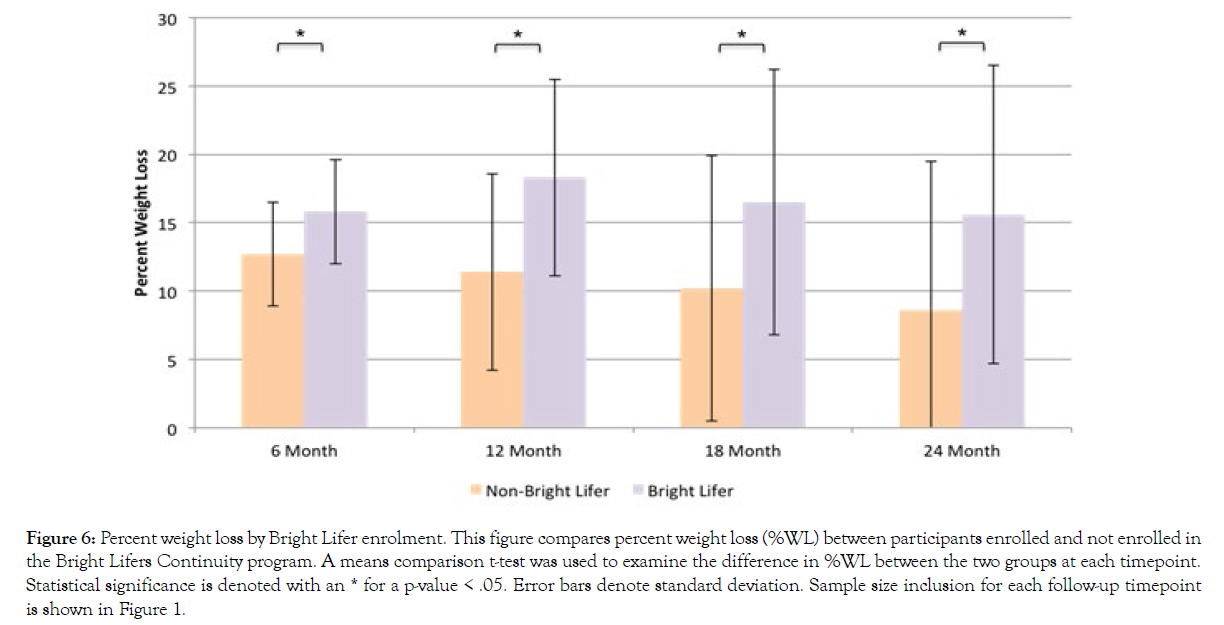
Figure 6: Percent weight loss by Bright Lifer enrolment. This figure compares percent weight loss (%WL) between participants enrolled and no t enrolled in the Bright Lifers Continuity program. A means comparison t-test was used to examine the difference in %WL between the two groups at each timepoint. Statistical significance is denoted with an * for a p-value < .05. Error bars denote standard deviation. Sample size inclusion for each follow-up timepoint is shown in Figure 1.
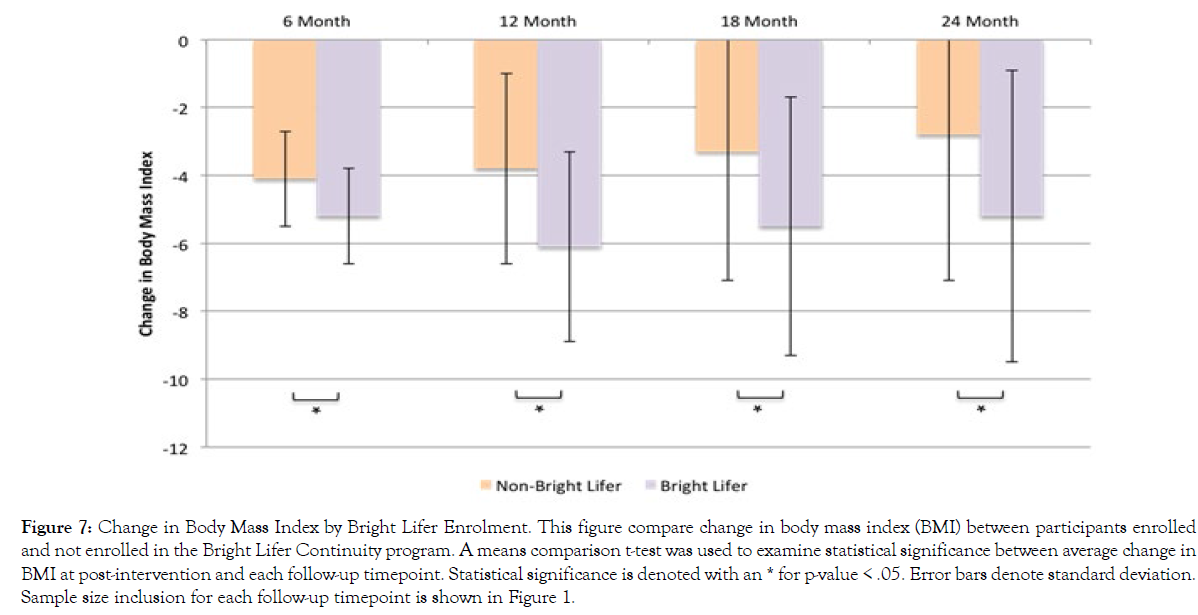
Figure 7: Change in Body Mass Index by Bright Lifer Enrolment. This figure compare change in body mass index (BMI) between participants enrolled and not enrolled in the Bright Lifer Continuity program. A means comparison t-test was used to examine statistical significance between average change in BMI at post-intervention and each follow-up timepoint. Statistical significance is denoted with an * for p-value < .05. Error bars denote standard deviation. Sample size inclusion for each follow-up timepoint is shown in Figure 1.
To compare %WL and BMI change across follow-up surveys, we used the restricted sample (which included only participants who completed the pre-intervention survey and all follow-up surveys). As stated previously and shown in Table 1, demographic characteristics of the restricted sample closely mirror the total sample. On average, participants in the restricted sample experienced 9.2%WL following the BLE:BC program. This increased to 16.1%WL at 6 months and 17.4%WL at 12 months, then decreased slightly to 15.7%WL at 18 months and 14.6%WL at 24 months. Similarly, participants saw an average 2.9 decrease in BMI at the end of the BLE:BC program, 5.2 decrease at 6 months, 5.6 decrease at 12 months, then 5.1 decrease at 18 months, and 4.7 decrease at 24 months.
Figures 8 and 9 present %WL and BMI change in the restricted sample, stratified by Bright Lifers enrollment. Among the 238 individuals included in the restricted sample, 188 enrolled in the Bright Lifers program. Repeated measures ANOVA was used to examine (a) whether there were any differences across all time points and (b) if there was a significant difference between non-Bright Lifers and Bright Lifers at each time point. Results show that the change across time for %WL was significant (F(4, 233)=8.23, p<.001). The effect of time varied, depending upon Bright Lifers enrollment (F(4, 233)=2.74, p=.030). Although there were no significant differences in %WL by Bright Lifers enrollment at post-intervention (t(236)=0.14, p=.888) and 6-month follow-up (t(236)=0.90, p=0.367), individuals who enrolled in the Bright Lifers program experienced greater %WL at 12 months (t(236)=2.23, p=.027), 18 months (t(236)=2.63, p=.009), and 24 months (t(236)=3.08, p=.002).
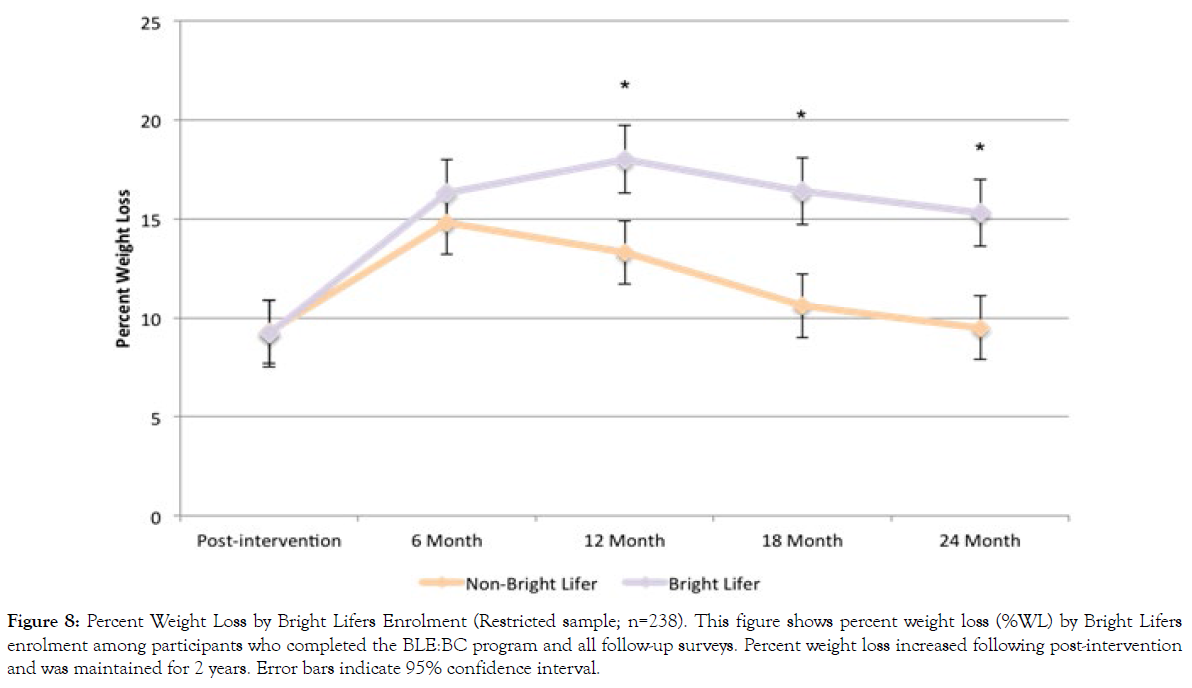
Figure 8: Percent Weight Loss by Bright Lifers Enrolment (Restricted sample; n=238). This figure shows percent weight loss (%WL) by Bright Lifers enrolment among participants who completed the BLE:BC program and all follow-up surveys. Percent weight loss increased following post-intervention and was maintained for 2 years. Error bars indicate 95% confidence interval.
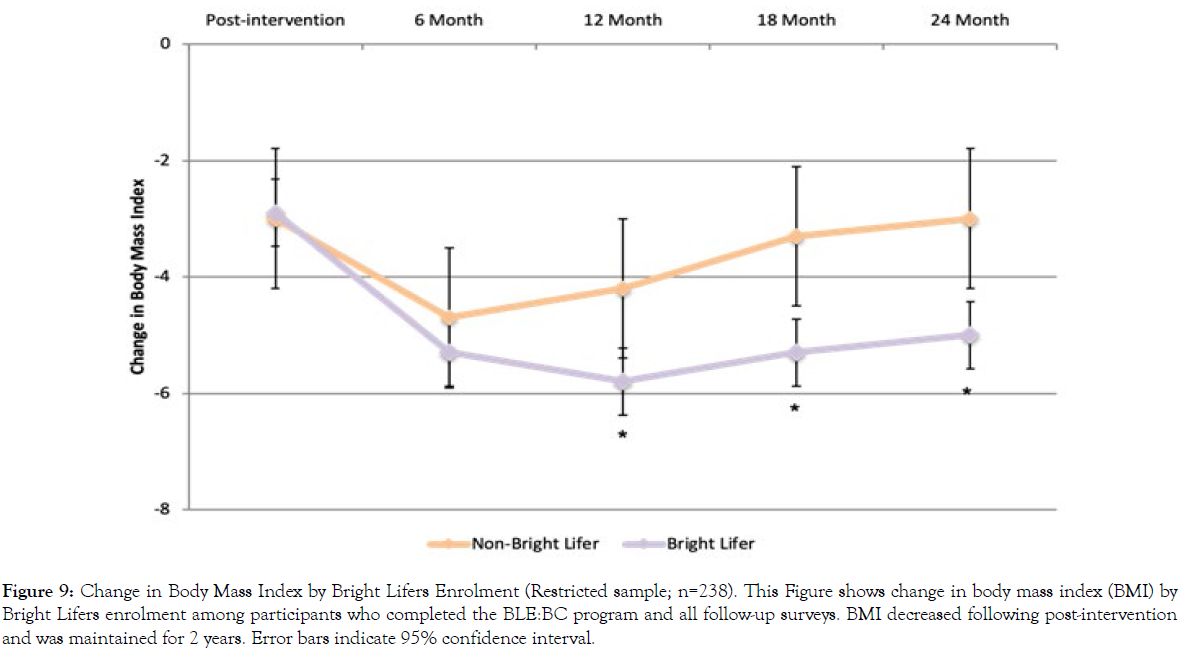
Figure 9: Change in Body Mass Index by Bright Lifers Enrolment (Restricted sample; n=238). This Figure shows change in body mass index (BMI) by Bright Lifers enrolment among participants who completed the BLE:BC program and all follow-up surveys. BMI decreased following post-intervention and was maintained for 2 years. Error bars indicate 95% confidence interval.
Results were similar for BMI change. They showed significant changes in BMI across time for all participants (F(4, 233)=5.48, p<.001). The effect of time varied, depending on Bright Lifers enrollment (F(4, 233)=2.24, p=.041). There was no significant difference in BMI change at post-intervention (t(236)=.22, p=.823) and 6-month follow-up (t(236)=.94, p=.350). However, individuals who enrolled in the Bright Lifers program experienced greater reductions in BMI at 12 months (t(236)=2.04, p=.043), 18 months (t(236)=2.50, p=.013), and 24 months (t(236)=2.40, p=.017).
Discussion
This study evaluated long-term weight loss and BMI change among participants in the BLE:BC program. The results support the efficacy of the BLE:BC for long-term weight loss and weight loss maintenance. Study participants in the total and restricted samples reported, on average, well beyond clinically-significant weight loss (>5%WL) at all time points. Additionally, participants who enrolled in the Bright Lifers continuity program following completion of the BLE:BC program experienced significantly greater weight loss and BMI reduction at 6 months, and at every subsequent time point, than those who opted not to receive ongoing support.
Results of this evaluation study carry important implications for research on commercial telehealth weight loss programs, food addiction and abstinence-based treatment, and the Bright Line Eating program. First, no prior study of a commercial telehealth weight loss program has shown positive results of long-term weight loss and weight loss maintenance of this magnitude. For instance, prior evaluations found an average of 0.9%WL at 16 weeks and 1.1%WL at 1 year for the eDiets.com program [9], 2.3%WL for the Biggest Loser Club at 12 weeks, and 3.3%WL for an enhanced version of the Biggest Loser Club [10]. Additionally, studies examining change in body mass index (BMI) found that BMI decreased by 1.2, on average, among participants in the Lose It! application at 6 months [11]; by an average of 0.7 for the basic version of the Biggest Loser Club at 12 weeks; and by an average of 1.0 for the enhanced version of the Biggest Loser Club at 12 weeks [10]. In contrast, the current study of the BLE:BC program found an average of 7.9%WL and 2.6 reduction in BMI immediately following the 8-week program and, on average, none of that weight was regained. Rather, participants continued losing weight after the Boot Camp and at the end of two years, sustained an average 14.3% WL and 4.7 reduction in BMI, nearly three times the accepted indicator for clinically significant weight loss.
Second, the results expand the literature on commercial weight loss programs. Numerous publications document the results of clinical and observational studies of weight loss interventions. However, surprisingly and unfortunately, commercial programs remain understudied. Given the extensive market for commercial weight loss programs and the number of individuals whom these programs affect, it remains critically important for researchers to fill this gap in the literature.
Third, the results of this study showed greater efficacy of the BLE:BC program among individuals who began the program with higher baseline weight status, which suggests a possible treatment avenue for individuals with obesity, especially those who don’t qualify for bariatric surgery.
Fourth, these results occurred within a food addiction framework, among participants guided to abstain completely from all sugar and flour. Abstinence-based approaches to weight loss and food addiction treatment are rare and have not yet been studied scientifically [13]. These results encourage further research on the efficacy of abstinence-based interventions within a food addiction framework.
Finally, obesity continues to carry significant public health implications, as it has been routinely shown to be associated with several leading causes of mortality including heart disease, cancer, and diabetes [1,2]. Moreover, as countries across the globe manage the impact of the COVID-19 pandemic, persons with obesity-- particularly severe obesity--have been disproportionately affected. As a result, it is paramount to evaluate weight loss programs and assess novel methods for encouraging weight loss and weight management. It’s especially important to rigorously evaluate commercial weight loss programs, given the fact that they constitute a $3.7 billion industry.
Limitations
Although the results of this evaluation show significant, positive weight-loss outcomes for the BLE:BC program, we acknowledge several limitations. First, survey participation was entirely voluntary, introducing selection bias. Specifically, individuals who lost more weight and/or who were more motivated to lose weight may have been more likely to complete follow-up surveys and to continue participation in the BLE:BC research program. However, rates of attrition in this sample, as shown in Figures 1 and 2, mirror that of long-term observational studies for clinical weight loss programs [15-17] and commercial weight loss programs [5,7].
Second, the observational case series research design of this study may introduce bias, because of the lack of a control group. Further research is needed to control for this bias by rigorously evaluating the impact of the BLE:BC program against a comparison group. Although an experimental randomized control trial is the gold standard of efficacy research, conducting a randomized control trial for commercial weight loss programs is not often feasible. Given that fact, results of observational case series designs are still necessary to assess the impact of commercial program designs on participant weight loss.
Third, the homogeneity of the sample-which may occur in part because of the cost of the BLE program-may produce sample bias and limit the generalizability of results. In other words, although the BLE:BC program was shown to produce significant weight loss for participants, the results may be generalizable only to participants with the same demographic characteristics as this sample (e.g., female, white, high socioeconomic status). However, according to data on the U.S. weight loss market, the demographic characteristics of this study sample do resemble the demographics of the broader population of commercial weight loss participants [18]. That increases confidence that the results of this study can be generalized to the population that typically participates in commercial weight loss. Further research is needed to understand whether these results hold for other demographic groups.
Conclusion
As commercial telehealth weight loss programs proliferate, it becomes increasingly important to evaluate their efficacy scientifically. This study demonstrates the food addiction recovery model can be highly effective in long-term weight management. Bright Line Eating offers a stringent but simple program that can produce substantial weight loss. In fact, it is the only commercial telehealth weight loss program that has been demonstrated empirically to produce clinically significant weight loss, on average, across time. Confirming the program’s efficacy as a sustainable weight loss option will require further analyses of participants’ long-term outcomes.
REFERENCES
- Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. Jama. 2013;309(1):71-82.
- Khaodhiar L, McCowen KC, Blackburn GL. Obesity and its comorbid conditions. Clin Cornerstone. 1999;2(3):17-31.
- Dietz W, Santos-Burgoa C. Obesity and its Implications for COVID-19 Mortality. Obesity. 2020;28(6):1005-1005.
- Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014; 63(25 Part B):2985-3023.
- Sarwer DB, Green AVS, Vetter ML, Wadden TA. Behavior therapy for obesity: where are we now? Curr Opinion Endocrinol Diabetes Obesity. 2009;16(5):347-352.
- https://www.marketresearch.com/Marketdata-Enterprises-Inc-v416/Weight-Loss-Diet-Control-12225125/
- Tsai AG, Wadden TA. Systematic review: An evaluation of major commercial weight loss programs in the United States. Ann Intern Med. 2005;142(1):56-66.
- Gudzune KA, Doshi RS, Mehta AK, Chaudhry ZW, Jacobs DK, Vakil RM, et al. Efficacy of commercial weight-loss programs: an updated systematic review. Ann Intern Med. 2015;162(7):501-512.
- Womble LG, Wadden TA, McGuckin BG, Sargent SL, Rothman RA, Krauthamer-Ewing ES. A randomized controlled trial of a commercial internet weight loss program. Obes Res. 2014;12(6):1011-1018.
- Collins CE, Morgan PJ, Jones P, Fletcher K, Martin J, Aguiar EJ, et al. A 12-week commercial web-based weight-loss program for overweight and obese adults: randomized controlled trial comparing basic versus enhanced features. JMIR. 2012;14(2):e57.
- Allen JK, Stephens J, Dennison Himmelfarb CR, Stewart KJ, Hauck S. Randomized controlled pilot study testing use of smartphone technology for obesity treatment. J Obes. 2013;2013.
- Guan W, Thaw A, Grondhuis SN, Schaechter A. Evaluation of a commercial telehealth weight loss and management program. J Nutr Weight Loss. 2018;3(2):1-6.
- Ifland J, Marcus MT, Preuss HG. Processed food addiction: Foundations, assessment, and recovery. CRC Press, USA. 2017.
- Brownell KD, Gold MS. Food and addiction: A comprehensive handbook. Oxford University Press, USA. 2012.
- Cottone P, Moore CF, Sabino V, Koob GF. Compulsive eating behavior and food addiction: Emerging pathological constructs. Academic Press, USA. 2019.
- Pirotta S, Joham A, Hochberg L, Moran L, Lim S, Hindle A, et al. Strategies to reduce attrition in weight loss interventions: A systematic review and meta-analysis. Obesity Rev. 2019;20(10):1400-1412.
- Moroshko I, Brennan L, O'Brien P. Predictors of dropout in weight loss interventions: a systematic review of the literature. Obesity Rev. 2011;12(11):912-934.
- Infinium Global Research. Global Weight Management and Wellbeing Products Market: Consumer Behavior Analysis by Countries, Buying Pattern Analysis, Demographics, Trends Analysis, Survey Findings and Results, Leading Companies and Their Market Strategies. Infinium Global Research. 2020.
Citation: Thompson SP, Thaw AK, Goetting MG, Guan W (2021) Bright Line Eating®: A Two-year Follow-up Evaluation of a Commercial Telehealth Weight Loss Program within an Abstinence-Based Food Addiction Framework. J Nutr Weight Loss. 6:3. 125.
Copyright: © 2021 Thompson SP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.