Review Article - (2020) Volume 8, Issue 2
Terpenoids form a substantial portion of chemical diversity in nature. The enormous terpenoid diversity of more than 80,000 compounds is supported by the multisubstrate and multiproduct nature of certain enzymes from the various terpene synthases and terpene cyclases. These highly versatile enzymes are not only able to accept multiple substrates in their active site, but also simultaneously catalyze multiple reactions to the resultant multiple products. Interestingly, apart from the substrates and catalytic mechanisms, multiple regulation factors are able to alter the product profile of multiproduct terpene synthases. Simple variations in cellular conditions by changes in metal cofactors, assay pH, temperature and substrate geometry lead to significant shifts in product profiles. Switch in substrate stereochemistry for multiproduct terpene synthases in some case shows enhanced biocatalysis and in others initiates even a novel cyclization cascade. Hence, organisms can get access to a greater chemodiversity and avoid the expensive process of developing new biocatalysts just by simple changes in the cellular environment. This possibility of modulating chemical diversity provides immobile plants in the same generation access to an enhanced chemical arsenal for defense and communication by simply altering cofactors, pH level, and temperature and substrate geometry.
Terpenoids; Biocatalysis; Polymers; Substrate isomers; Catalysis
Plants being immobile organisms do not have the ability to escape from hostile conditions. Plants adapt to environmental cues by physiological, morphological and metabolic changes due to restricted mobility. Their inherent phenotypic plasticity provides a unique feature to defend themselves against predators and environmental changes. A significant tool for plants to defend, adapt and handle complex communication among organisms is metabolic diversity. This metabolic diversity allows them to biosynthesize a vast number of natural products for their survival and growth [1-4]. Plants dynamically modify their metabolic profile depending on the multiple variations in environmental factors for which they have a complex network of adaptable metabolic pathways. The tremendous chemodiversity through secondary metabolites expands the adaptability of plants. For example, phytohormones regulate growth, phenolics and waxy cuticles acts as sunscreen, plant polymers like lignin and rubber provide support and wound healing. Other metabolites mediate interspecies interactions like seed dispersion, enticing pollinators and defend against predators [4-6].
Plant natural product diversity can be based on the promiscuity of the corresponding biosynthetic enzymes. The number of secondary metabolites and of the corresponding enzymes is continuously expanding through a restricted number of folds in these enzymes. These metabolic enzymes are assumed to have emerged through early gene duplication and further mutations, which enhanced the substrate plasticity and lowered catalytic activation energy barriers. This enzymatic evolution resulted in mechanistic plasticity resulting in individual enzymes catalyzing the biosynthesis of multiple reactions and a large number of products [4]. This inference is drawn from the study of mutant libraries from phylogenetic families of plant metabolic enzymes and directed evolution based on enzyme promiscuity [7-9]. Enzymes are usually considered to be “single product” due to their specificity for both products and substrates, however some of these biocatalysts possess exceptional regio- and stereoselectivity for even multiproduct biosynthesis. This is particularly true in case of the chemodiversity of the largest class of natural products, the terpenoids [10-13]. This enormous structural wealth results from the combinatorial action of terpene synthases and cyclases. Most of the terpenoids are produced by plants and serve as precursors of many essential compounds like vitamins, hormones and medicines, apart from providing plants with their distinct fragrance and flavor properties. Essentially all terpenoids are derived by the condensation of multiple units of isopentenyl diphosphate (IDP) and its isomer dimethylallyl diphosphate (DMAPP) by prenyl converting enzymes, further conjugation or cyclization steps significantly enhance the chemodiversity in nature. As outlined in Figure 1 these classical enzymes combine and convert only a small number of acyclic precursors into the ca. 80,000 terpenoids found in nature [14].
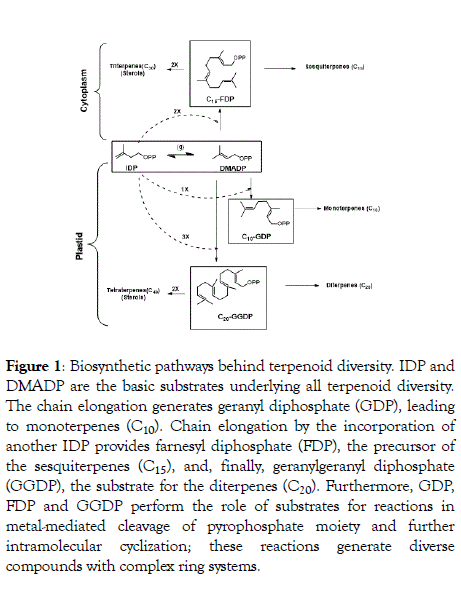
Figure 1: Biosynthetic pathways behind terpenoid diversity. IDP and DMADP are the basic substrates underlying all terpenoid diversity. The chain elongation generates geranyl diphosphate (GDP), leading to monoterpenes (C10). Chain elongation by the incorporation of another IDP provides farnesyl diphosphate (FDP), the precursor of the sesquiterpenes (C15), and, finally, geranylgeranyl diphosphate (GGDP), the substrate for the diterpenes (C20). Furthermore, GDP, FDP and GGDP perform the role of substrates for reactions in metal-mediated cleavage of pyrophosphate moiety and further intramolecular cyclization; these reactions generate diverse compounds with complex ring systems.
Unlike the well-known single product enzymes, in terpenoid biosynthesis also multiproduct terpene synthases transform individual substrates into a larger number of structurally diverse compounds. Moreover, these enzymes can display multisubstrate behavior by accepting substrates of different chain lengths to generate an even broader range of terpenoid skeletons. As an example, the γ-humulene synthase from Abies grandis generates 52 different sesquiterpenes from farnesyl diphosphate (FDP) [15-18] and certain diterpene synthases (diTPSs) also accept alternative C20 substrates to produce multiple products [19-23].
Most organisms, especially plants contain a large number of functional TPS that all together produce an even larger amount of products [24]. For example, the grand fir, Abies grandis, contains seven mono-TPS genes, three sesqui-TPSs, and one di- TPS gene, [17,18,25-28] Norway spruce, Picea abies, contains five mono-TPS genes, three sesqui-TPS genes, and two di-TPS genes, all of them were functionally characterized [29,30]. On the other hand, more than 40 TPS genes are present in the sequenced Arabidopsis thaliana genome [31]. Genome-wide analysis of the TPS families suggests that functionally diverse TPS genes arose from gene duplication, gene mutations, and functional diversification during the evolution. The plasticity of multiple gene based terpenoid defense is an important adaptive trait of conifers that interact on several trophic levels with predators like insects. Allocation of terpenoids with appropriate spatial and temporal patterns for defense is very important for the flexibility of a conifer’s defensive arsenal [32-41].
Here we focus on the role of multiproduct synthases, multisubstrate synthases, and the effects of reaction conditions in modulating the terpene synthase activity, such as metal cofactors, stereochemistry of the substrates and pH level.
Particularly complex terpenoid bouquets originate from plants and often serve as a direct and indirect defense against herbivores. Major factors behind this enormous diversity are on one hand the presence of a large number of synthases in the genomes of higher plants, on the other hand the existence of multiproduct enzymes. A well-studied example from Medicago truncatula is shown in Figure 2 which converts a single substrate (FDP) into a bouquet of cyclic and acyclic products [42-49].
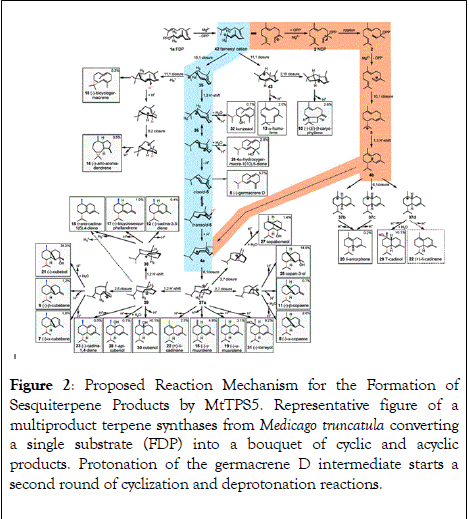
Figure 2: Proposed Reaction Mechanism for the Formation of Sesquiterpene Products by MtTPS5. Representative figure of a multiproduct terpene synthases from Medicago truncatula converting a single substrate (FDP) into a bouquet of cyclic and acyclic products. Protonation of the germacrene D intermediate starts a second round of cyclization and deprotonation reactions.
Many terpene synthases are inherently known to be multiproduct in nature, almost half of identified mono- and sesquiterpene synthases produce several products to be classified as multiproduct synthases [40,44]. This multiproduct and multisubstrate nature reflects the nature’s tendency to utilize existing mechanisms to maximize the chemical diversity by using the least expensive biosynthetic routes [36,45,46]. This multisubstrate nature resulting from enzymatic promiscuity can easily characterized by rapid improvements in detection techniques [47,48]. δ-selinene synthase with 52 products and γ-humulene synthase with 34 products from Abies grandis hold the current record for the biosynthesis of multiple sesquiterpenes from farnesyl diphosphate (FDP) [18,27,42,43].
Terpene synthases/cyclases use a series of consecutive chemical reactions after carbocation formation to initiate product cascade protonations and deprotonations, hydride or alkyl shifts, ring closures, alkene-cation cyclizations, and water quenching. Pathway prediction has always been of great interest in terpenoid chemistry, especially due to complexity of multiproduct terpene synthases (Figure 2). The studies so far show that product specificity is achieved by stabilization of transitional carbocations through spatial positioning of aromatic side chains in corresponding amino acids [41].
Several terpene synthases have the tendency to form multiple products, but the diversity of products depends on the individual terpene synthase with many known for their strict control on the stereospecificity of the products and others exerting low control on the reaction cascade by producing different terpenes. The catalytic cascade starts by the generation of a highly reactive carbocation intermediate, which further undergoes a series of different cyclizations, hydride shifts, and other rearrangements. The catalytic cascade is initiated by a metal cation mediated cleavage of the diphosphate anion or substrate protonation, and termination steps are usually proton abstraction or quenching by addition of water. During this cascade at several levels exist multiple possibilities of branching and termination, resulting in TPSs being multiproduct in nature. However, there also exists a vast majority of monoterpene and sesquiterpene synthases that have evolved into single product enzymes. The high enantiomeric excess of the different products of MtTPS5 suggests complete catalytic control of enzymatic cascade leading to multiple products. A list of multiproduct terpene synthases and their product diversity is available in a recent review by Vattekkatte [49].
Each terpene synthase has its own individual way of catalysing the biosynthesis of multiple products and follows its own pathway that is highly stereospecific in some cases. The γ- humulene synthase of Abies grandis produces 52 different products from a single substrate C15 FDP (Figure 3). The cascade is initiated at the entrance of the active site by two DDxxD motifs with metal binding. In the absence of a DDxxD motif, the biosynthesis resulted in fewer products indicating the presence of two varying conformations with substrate binding in the motifs leading to multiple products. The NSE/DTE motif present at an identical location as the second DDxxD motif in other terpene synthases has shown an increase in formation of multiple products [40]. The multiproduct sesquiterpene synthase MtTPS5 from Medicago truncatula biosynthesizes 27 products, each of them almost optically pure ( ≥ 98% ee) belonging to a common structural group from farnesyl diphosphate (FDP). Garms et al., using a series of incubation experiments with deuterium-containing substrates were able to elucidate the reaction pathway leading up to the formation of 27 products; the entire stereochemical reaction cascade was rebuilt by determining the absolute configuration of individual products and the initial conformations of the substrate [49,50]. A single amino acid mutation had a dramatic effect on the product profile of the MtTPS5; the substitution of a tyrosine by a phenylalanine (Y526F) aborts the pathway, since protonation of the key intermediate germacrene D is essential to start a second sequence of cationic rearrangements and terminal proton losses or hydroxylations (Figure 2). The stereospecific course seen in MtTPS5 is not observed in case of the TPS4 multiproduct terpene synthase from Zea mays or the trichodiene synthase from Fusarium sporotrichioides leading up to racemic and diastereomeric products [51-53]. Cop4 from Coprinus cinereus is also a multiproduct enzyme which converts FDP into multiple terpenoids, including (−)-germacrene D and cubebol [54-56]. Sandalwood which has huge commercial significance and comprises a monoterpene synthase, namely SamonoTPS1, which produces a mixture of (+)-α-terpineol and (−)-limonene, along with linalool, myrcene, (−)-α-pinene, (+)-sabinene and geraniol. SasesquiTPS1 as corresponding sesquiterpene synthase generates germacrene-D-4-ol and helminthogermacrene along with α-bulnesene, γ-muurolene, α- and β-selinenes, as well as several other minor bicyclic compounds (Figure 3) [57,58].
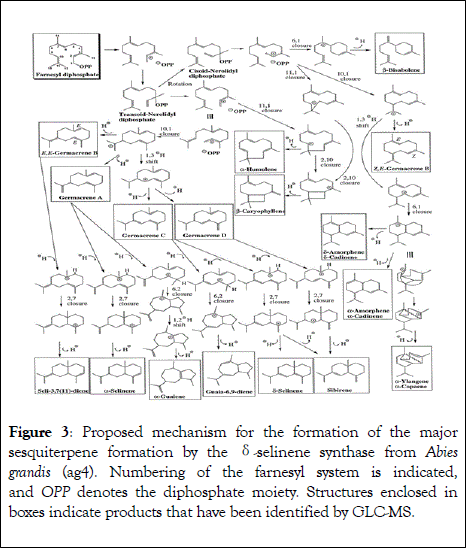
Figure 3: Proposed mechanism for the formation of the major sesquiterpene formation by the δ-selinene synthase from Abies grandis (ag4). Numbering of the farnesyl system is indicated, and OPP denotes the diphosphate moiety. Structures enclosed in boxes indicate products that have been identified by GLC-MS.
TPS4 from maize Zea mays is also a multiproduct synthase, as well as a multisubstrate terpene synthase, it generates 14 terpenoids of which 12 are produced in minor quantities, major products are 7-epi-sesquithujene and β-bisabolene. The active site architecture and the multiple product formation in TPS4 were correlated using modelling studies and models based on known terpene crystal structure (Figure 4) [52]. Models of the TPS4 active site with natural substrate FDP inside and docking simulations were used to predict the structural basis of the biosynthetic cascade. These models revealed the presence of two different pockets in the active site, with each controlling separate reaction steps leading up to change in conformation of the carbocationic intermediate [59]. The flexibility of possible conformations in the active site that can lead to various carbocationic intermediates is a major contributor to the product spectrum. Conversely, strict conformational integrity has to be ensured in the enzymatic cavity to prevent premature quenching of highly reactive carbocation intermediates by solvent or coproduct PPi.
The multiple products may suggest a lack of enzymatic control over the reaction cascade, but the MtTPS5 from Medicago truncatula shows a high stereospecificity in the end products and points to a conformationally controlled access of nucleophiles to the carbocation prior to the termination step for each product. The short time spans for carbocationic cyclizations restrict the possibility for major conformational variations [60,61]. At the same time they stabilize intermediates by long-range electrostatic interactions, e.g., with PPi, peptide backbone carbonyl groups, or the electrons of aromatic residues in the active site. Cocrystallization studies with the trichodiene synthase and intermediate azaanalogs showed in detail that the binding conformations and orientations of e.g. R-azabisabolene to the trichodiene synthases do not correspond to binding conformations required for product formation. Since the binding conformations and orientations of diverse substrate and carbocation analogues to other cyclases such as 5-epiaristolochene synthase and bornyl diphosphate synthase generally do not correspond to catalytically productive complexes, it is assumed that the formation of transient carbocation intermediates in terpene cyclization reactions is generally under kinetic rather than thermodynamic control [38,60,62].
Multi-substrate enzymes form different products with different substrates, allowing the modification of the terpene pattern by varying substrate specificity and corresponding availability. Many plants lack the possibility of terpene storage. Using the existing enzymatic pool they can achieve rapid alteration of the product profile by variation in the substrate pool [63]. The current consensus on the localization of different terpene synthases is that hemiterpene, monoterpene, and diterpene synthases are limited to plastids utilizing substrates from the MEP pathway, while sesquiterpenes are localized in the cytosol and are fed by the MVA pathway [1,64,65]. With common starting substrates in both MVA and MEP pathways there has been notable progress in understanding of subcellular localization of terpenoids and cross talk between the two pathways with exchange of substrates and products. The importance of multisubstrate terpene synthases can be seen with examples of the biosynthesis of monoterpenes by multi-substrate sesquiterpene enzymes in the cytosol [66-69].
Multi-substrate nature of a terpene synthase was observed in the (2E,6E)-β-farnesene synthase from Mentha × piperita, which produces mainly (E,E)-β-farnesene (85%) and minor amounts of (Z)-β-farnesene (8%) and δ-cadinene (5%) [70]. This enzyme lacks an N-terminal transit peptide, and also uses GDP as substrate and to produce limonene (48%), terpinolene (15%), and acyclic myrcene (15%). More than 40 examples of multisubstrate plant terpene synthases have been described by now [63]. The α-bisabolene synthase from Abies grandis generates α-bisabolene with FDP and (+)-limonene with GDP as the substrate. Interestingly, this synthase has a greater sequence similarity to other sesquiterpene synthases from A. grandis (δ-selinene synthase and γ-humulene synthase) than to corresponding monoterpene synthases. A germacrene C synthase from a clone of Solanum lycopersicum uses FDP to generate germacrene C (64%), germacrene A (18%), germacrene B (11%), and germacrene D (7%), but only limonene with GDP as the precursor [71].
Both recombinant terpenoid synthases TPS4-B73 and TPS5- Delprim from Zea mays accept and convert (2E)-GDP and (2E, 6E)-FDP into families of monoterpene and sesquiterpene products.72 Both enzymes formed the same complex mixture of sesquiterpenes from (2E,6E)-FDP but with different proportions of products (Figure 4A-4C). The mixtures reflected the sesquiterpene blends observed in the varieties B73 and Delprim, respectively.
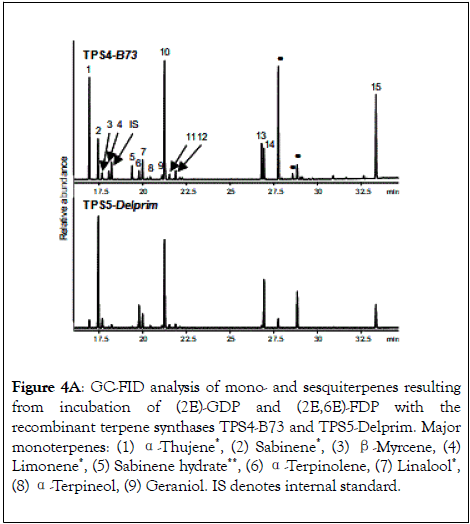
Figure 4A: GC-FID analysis of mono- and sesquiterpenes resulting from incubation of (2E)-GDP and (2E,6E)-FDP with the recombinant terpene synthases TPS4-B73 and TPS5-Delprim. Major monoterpenes: (1) α-Thujene*, (2) Sabinene*, (3) β-Myrcene, (4) Limonene*, (5) Sabinene hydrate**, (6) α-Terpinolene, (7) Linalool*, (8) α-Terpineol, (9) Geraniol. IS denotes internal standard.
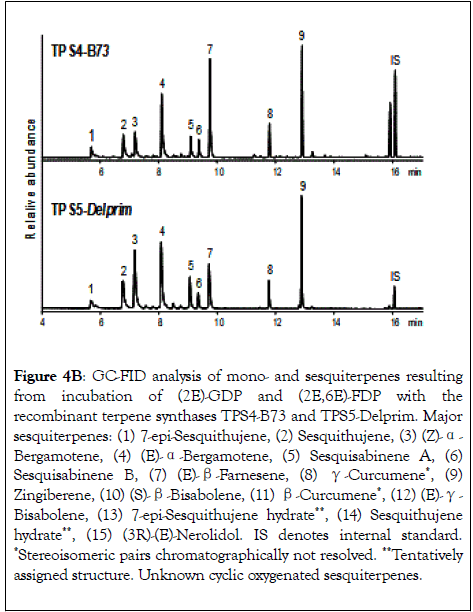
Figure 4B: GC-FID analysis of mono- and sesquiterpenes resulting from incubation of (2E)-GDP and (2E,6E)-FDP with the recombinant terpene synthases TPS4-B73 and TPS5-Delprim. Major sesquiterpenes: (1) 7-epi-Sesquithujene, (2) Sesquithujene, (3) (Z)-α- Bergamotene, (4) (E)-α-Bergamotene, (5) Sesquisabinene A, (6) Sesquisabinene B, (7) (E)-β-Farnesene, (8) γ-Curcumene*, (9) Zingiberene, (10) (S)-β-Bisabolene, (11) β-Curcumene*, (12) (E)-γ- Bisabolene, (13) 7-epi-Sesquithujene hydrate**, (14) Sesquithujene hydrate**, (15) (3R)-(E)-Nerolidol. IS denotes internal standard. *Stereoisomeric pairs chromatographically not resolved. **Tentatively assigned structure. Unknown cyclic oxygenated sesquiterpenes.
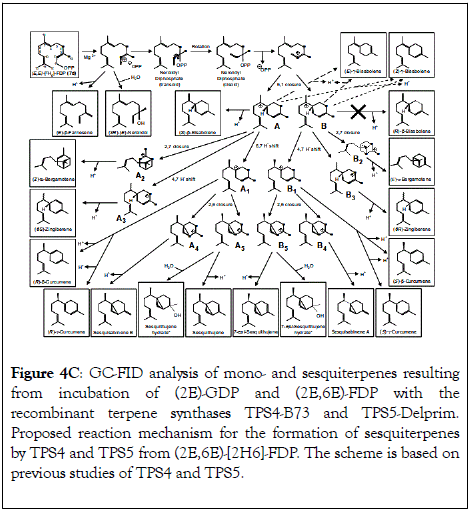
Figure 4C: GC-FID analysis of mono- and sesquiterpenes resulting from incubation of (2E)-GDP and (2E,6E)-FDP with the recombinant terpene synthases TPS4-B73 and TPS5-Delprim. Proposed reaction mechanism for the formation of sesquiterpenes by TPS4 and TPS5 from (2E,6E)-[2H6]-FDP. The scheme is based on previous studies of TPS4 and TPS5.
The multisubstrate nature of these terpene synthases also extends to simultaneous production of hemi- and monoterpenes (C5 and C10 substrates), mono-, sesqui,- and diterpenes(C10-C20 substrates), along with sesqui- and diterpenes (C15 and C20 substrates). A comprehensive list of multisubstrate enzymes and their relative activity with different substrates is given in a recent review by Pazouki [63]. The presence of a putative transit peptide, having generally a low homology, makes the actual subcellular targeting difficult [8]. The multi-substrate capacity is more widespread across TPS families in multiple species than previously estimated, nine multi-substrate TPS enzymes have been identified in Vitis vinifera, two generate simultaneous C10/C15/C20-terpenes, while the others produce C10/C15- volatiles. In Santalum species, seven confirmed C10/C15 multisubstrate TPSs are known [57,58,72,73]. However, TPSs in general cannot be classified as multi-substrate synthases, steric limitations and active center configurations and overall flexibility of protein structure restricts the usage of multiple substrates. Also, important to note is the significant variation in affinity for different substrates and product specificity. Comparison of two C10/C15/C20 TPSs, one from Phaseolus lunatus TPS (PlTPS2) and the MtTPS3 from Medicago truncatula, showed completely different substrate specificities. Incubation with equimolar substrate mixtures of GDP, FDP, and GGDP, the largest product formation for PlTPS2 was observed for linalool (C10), second for (E)-nerolidol (C15) and then least for (E,E)-geranyllinalool (C20) [74]. In contrast, with the MtTPS3 from Medicago truncatula the highest rate of terpenoid formation was noticed for (E)-nerolidol (C15), then (E,E)-geranyllinalool (C20) and lowest for the monoterpene linalool (C10) [75]. Such affinity differences among substrates indicate the importance of the active center and the tertiary structure of proteins which build the capacity for multi-substrate enzymes. However, the multisubstrate nature of terpene synthase provides the organism an opportunity to expand the terpenoid arsenal and thus, favor synthesis of terpenoids through environment-controlled pathways.
Terpene synthases involved in the biosynthesis of monoterpenes and sesquiterpenes are known to be multiproduct in nature and for being promiscuous with their multisubstrate nature. Diterpene synthases leading to C20 diterpenes are known for their cyclization complexity and are generally considered to be single product biocatalysts. However, levopimaradiene/ abietadiene synthase and isopimaradiene synthase from Norway spruce (Picea abies), are the major source of hydrocarbons for the defensive diterpene resin acids. Interestingly, both of these enzymes share 91% sequence similarity in amino acids, but one catalyzes the formation of a single product with high specificity and the other acts as a multiproduct diterpene synthase leading to an entirely different product profile. Levopimaradiene/ abietadiene synthase shows interesting tendencies with genetic mutations, a single amino acid switch transforms the enzyme into generating just isopimaradiene and sandaracopimaradiene devoid of any usual diterpene products. Enhancing the amino acid exchange to four mutations completely reversed the diterpene profiles for both synthases while maintaining catalytic turnovers comparable to the wild-type [65]. Hence, these mutational studies show the versatility of diterpene synthases, where a switch is possible between multiple products and novel products with single to four amino acid mutations. Multisubstrate promiscuity was examined with a modular metabolic engineering system of class I diterpene synthases. Surprisingly, bacterial and plant diterpene synthases converted many precursors and exhibited extreme promiscuity. This multisubstrate promiscuity is well suited for combinatorial biosynthesis, with high yields observed for non-native enzymatic combinations. These enzymatic combinations have led to diterpenes previously biosynthetically uncharacterized. It provides a unique access, to the assembly of extended pathways to generate non-natural and previously inaccessible diterpenoids [21].
Detailed investigation of terpene synthase structure-function relationship is critical for correlation of enzymatic architecture that catalyzes the multiple product formation. Lack of available multiproduct terpene synthase crystal structures and definitive structural assignments is a major impeding in understanding the multiproduct phenomenon. It is critical to understand the amino acid residues that stabilize the individual steps of the reaction cascade by structural elucidation via available cocrystallization candidates [76-78]. Furthermore the knowledge of key amino acids governing the carbon flux in distinct directions can be used for tailoring enzymatic catalysis accordingly. Available modelling studies suggest that the multiproduct formation depends on conformational variations of the substrate during the initial stages of the reaction. Thus, it will be of great interest to observe the enzymatic response to intermediate mimics such as substrate isomers on incubation with multiproduct terpene synthases. A major determinant for product selectivity is the degree of conformational flexibility that the substrate enjoys in the active site of a terpene synthase. Hence, the most interesting comparison could be made with sesquiterpene synthases, Cop4 and Cop6 from Coprinus cinereus, Cop6 with its narrow binding pocket is a high fidelity enzyme, while the large cavity of Cop4 yields multiple cyclization products. Consequently, the fidelity of the cyclization reaction and multiple products may be achieved by modification of the conformational flexibility and the active site fit of the bound substrate. This alteration can be achieved by protein engineering or modifying the physical environment of an enzyme. There are many examples of protein engineering to explore the catalytic promiscuity of terpene synthases [79,80].
(E)-isomers as natural substrates for terpene synthases was a generally accepted fact until it was challenged by a tomato monoterpene synthase that preferred neryl diphosphate (the (Z)- GDP) over (E)-GDP [81]. Further support for the relevance of (Z)-substrates was given by a sesquiterpene synthase from wild tomato Solanum habrochaites that preferred (2Z,6Z)-FDP as a substrate over the usual (2E,6E)-FDP [82]. The reports about a (2Z,6E)-FDP synthase from Mycobacterium tuberculosis and, more recently, the isolation of novel terpene synthase evolving from trans-isoprenyl diphosphate synthases in the striped flea beetle indicate a more widespread prevalence of isomeric prenyl diphosphates as natural substrates [83-85]. As outlined in Figure 5 the MtTPS5 from Medicago truncatula simultaneously uses (2E, 6E)-FDP and nerolidyl diphosphate (NDP) as substrates, converting them into different families of sesqiterpemes [86,87].
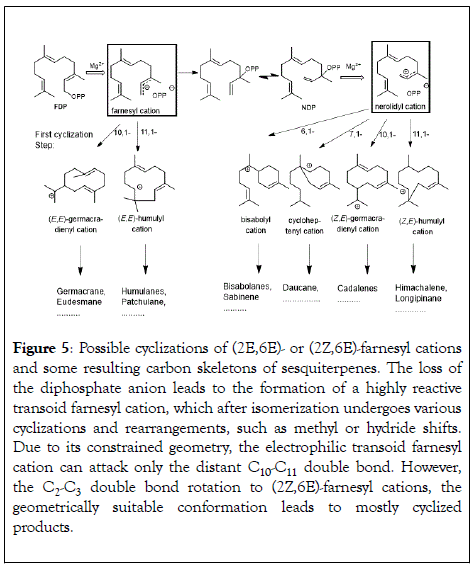
Figure 5: Possible cyclizations of (2E,6E)- or (2Z,6E)-farnesyl cations and some resulting carbon skeletons of sesquiterpenes. The loss of the diphosphate anion leads to the formation of a highly reactive transoid farnesyl cation, which after isomerization undergoes various cyclizations and rearrangements, such as methyl or hydride shifts. Due to its constrained geometry, the electrophilic transoid farnesyl cation can attack only the distant C10-C11 double bond. However, the C2-C3 double bond rotation to (2Z,6E)-farnesyl cations, the geometrically suitable conformation leads to mostly cyclized products.
Multiproduct terpene synthases from maize TPS4-B73 and TPS5-Delprim, were incubated with various deuterium labelled isomers of natural substrates to study the kinetic isotope effects and observe effects of the double bond geometry. Hence (2Z)- [2-2H]- and [2,4,4,9,9,9-2H6]-geranyl diphosphates (GDP) and (2Z,6E)-[2-2H] and [2,4,4,13,13,13-2H6]-farnesyl diphosphates (FDP) were synthesized as substrate analogs to mimic presumptive reaction intermediates. Interestingly, acyclic products showed a remarkable decrease on comparison with products from unlabeled (2E)-GDP and (2E,6E)-FDP (Figure 6B). Additionally, TPS4 and TPS5 exhibited much higher turnover when incubated with (2Z)-substrates, due to the lack of kinetic isotope effects of primary deuterium atoms for terminating deprotonations and secondary deuterium atoms resulting from the hyperconjugation stabilization of the reaction intermediates (Figure 6). This observation of isotopically sensitive branching of product formation in maize supports the enzymatic biosynthesis of mono- and sesquiterpene volatiles from a shared carbocationic precursor for branched cascade in multiproduct synthases [51,86].
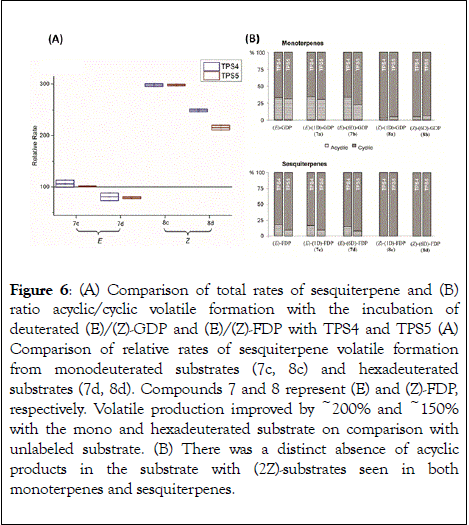
Figure 6: (A) Comparison of total rates of sesquiterpene and (B) ratio acyclic/cyclic volatile formation with the incubation of deuterated (E)/(Z)-GDP and (E)/(Z)-FDP with TPS4 and TPS5 (A) Comparison of relative rates of sesquiterpene volatile formation from monodeuterated substrates (7c, 8c) and hexadeuterated substrates (7d, 8d). Compounds 7 and 8 represent (E) and (Z)-FDP, respectively. Volatile production improved by ~200% and ~150% with the mono and hexadeuterated substrate on comparison with unlabeled substrate. (B) There was a distinct absence of acyclic products in the substrate with (2Z)-substrates seen in both monoterpenes and sesquiterpenes.
TPS4 and TPS5 from Zea mays showed major quantitative enhancements in enzymatic turnover with isomeric (Z)-substrates with identical product profile as compared with natural (E)- substrates [86]. In contrast, MtTPS5 enzyme from Medicago truncatula when probed for promiscuity with (2Z,6E)-FDP substrate showed substantial qualitative variations in product profile, involving the biosynthesis of a novel product profile with no identical products on comparison with (2E,6E)-FDP (Figure 5) [87]. The (Z)-substrate resulted in initiation of a novel cyclization pathway leading to carbocationic cascade of sesquiterpenes resulting in humulane, amorphene and himachalane based products (Figure 7). Determination of absolute configuration of each product, helped in reconstructing the exact stereochemical pathway. Moreover, observation of only one enantiomer of each product indicated the high stereospecificity and control of the biosynthetic cascade. The substrate promiscuity sometimes observed in terpene biosynthesis provides living organisms with access to optically pure chemical blends without the costly genetic optimization of available enzymes or new enzymes. The presence of these new products in natural volatiles of Medicago truncatula indicates the possible occurrence of this alternate cascade in nature.
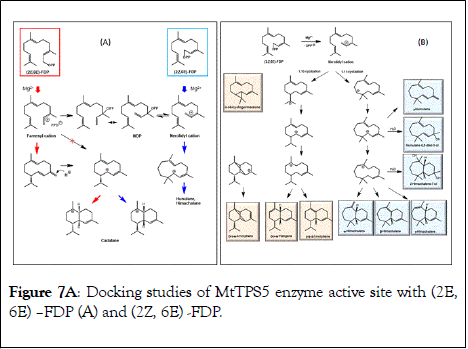
Figure 7A: Docking studies of MtTPS5 enzyme active site with (2E, 6E) –FDP (A) and (2Z, 6E) -FDP.
Docking studies were performed with (2E,6E)-FDP (Figure 7A) and (2Z,6E)-FDP (Figure 7B) in order to understand the major shift in product profile in the MtTPS5 enzyme with both the substrates. (2E,6E)-FDP (Figure 7A) substrate with cyclic conformations showed the C3 methyl group pointing in the direction of the postulated catalytic triad (D450, Y526, N530), responsible for the proton transfer involved in the formation of germacrene D. The mutagenesis and labeling studies were used to demonstrate the importance of the hydroxyl group of tyrosine (Y526) in proton transfer due to the proximity of the potential proton source and the acceptor [49]. Y526F mutant shifts to germacranes (80%) by the resultant reduction of the cadalanes (15% of the product profile). The critical part played by the hydroxyl group of tyrosine (Y526) leading to intermediate germacrene D is supported by increase in the precursor related products and a low deuterium incorporation in cadalane based products in D2O incubation assays. However, the tyrosine exchange by phenylalanine group does not completely deactivate the reprotonation step, indicating the presence of a water molecule as a Lewis acid [43,49]. In contrast, the in silico analysis of (2Z,6E)- FDP with MtTPS5 showed no conformations that justify the cyclized products, in which the methyl group at C3 was localized in the vicinity of Y526, consistent with D2Olabeling results with absence of labeled products. Out of total seven possible conformations that allowed cyclization to a 10- or 11- membered ring (C1-C10 and C1-C11), only one conformation led to the observed stereochemistry of the bridgehead hydrogen atoms of himachalane and amorphane (Figure 7B). In multiproduct terpene synthases accepting substrate isomers, these differences initiate an alternate cyclization cascade [50,87].
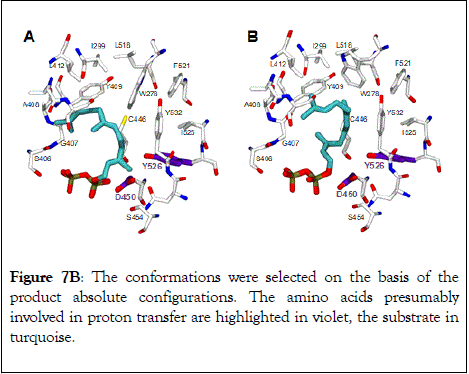
Figure 7B: The conformations were selected on the basis of the product absolute configurations. The amino acids presumably involved in proton transfer are highlighted in violet, the substrate in turquoise.
Isomeric prenyl diphosphates have been used to study the cyclization of all-trans-FDP to the cis-trans isomer of FDP (2Z,6E)- FDP and also as a cisoid intermediate mimic for the rotation of C2-C3 double bond of (2E,6E)-FDP by sesquiterpene synthases (Figure 3). Cop4 and Cop6 from Coprinus cinereus, were investigated with both geometric isomers for comparison of activity and conformation flexibility. In case of Cop6, the conversion of (2E,6E)-FDP continues through a (6R)-β- bisabolyl carbocation and while Cop4 through (2E,6E)- germacradienyl carbocation. However, in the case of (2Z,6E)- FDP as substrate, both Cop4 and Cop6, the cascade continues via the (6S)-β-bisabolene cation [56].
Sesquiterpene synthases from the liverwort Heteroscyphus planus converted (2E,6E)-FDP and (2Z,6E)-FDP to different isomeric cadinanes, cyclization cascade of the two substrates diverge only after 1,10-cyclization of the cisoid farnesyl cation intermediate [88]. The maize enzymes TPS4, TPS5 and the liverwort enzyme terpene synthases act identically on incubation with (2E,6E)- FDP and (2Z,6E)-FDP when it comes to generation and cyclization of the first cisoid allylic carbocation intermediate. Cop4 and Cop6 and MtTPS5 however, yield very different products with (2E,6E)-FDP and (2Z,6E)-FDP, the two Cop enzymes and MtTPS5 must bind the two geometrical isomers as different conformers resulting in the generation of opposite enantiomers of the first cyclic cation intermediate [56]. For comparison, as a control strictly trans-pathway specific germacrene A synthase NS1 from Nostoc sp. PCC 7120 can be used, whic88h ideally should not accept (2Z,6E)-FDP as a substrate [54]. As expected, the strictly trans-pathway specific enzyme NS1 expectedly does not convert (2Z,6E)-FDP because it either is unable to bind (2Z,6E)-FDP or to catalyze diphosphate cleavage.
Cop4, Cop6 and MtTPS5 showed significant qualitative changes in product profile, analogously this phenomenon is more widespread among other species. Germacrene A synthase in yarrow (Achillea millefolium) is an enzyme with mixed substrate specificity. Besides (2E,6E)-FDP, the AmGAS accepts both C10 GDP and NDP (neryl diphosphate) as substrates, whereas with GDP resulted in exclusively the formation of acyclic monoterpenes, and NDP in formation of cyclic monoterpenes [89]. Santalene synthase (SaSSy) from Santalum album accepts both isomers (2E,6E)-FDP, (2Z,6Z)-FDP and GDP as substrates. However, differently from AmGAS, sesquiterpene product mixture only differed moderately with (2E,6E)-FDP and (2Z,6Z)- FDP [58]. Several monoterpene synthases are known to use NDP instead of GDP, e.g., a tomato (S. lycopersicum) monoterpene β-phellandrene synthase expressed in glandular trichomes [81]. Sesquiterpene synthases that prefer (2Z,6Z)-FDP instead of the usual (2E,6E)-FDP, e.g. a santalene and bergamotene synthase in wild tomato (S. habrochaites), are known [82]. However, these cis-substrate using enzymes do not accept the trans-substrates. Thus, the capacity to use both the cisand trans-substrate isomers in the enzymes, AmGAS, SaSSy, MtTPS5 and Cop4 suggests a high plasticity of the enzyme’s active centers. Multi-substrate enzymes with their high active center plasticity are less frequently tested with NDP and (2Z,6Z)- FDP than GDP and (2E,6E)-FDP. Hence there exists a wide possibility of using cis-substrates across TPSs. Substrate isomers provide terpene synthases with the opportunity to utilize the existing enzymatic architecture to modulate the turnover advantage and initiate novel chemistry. The upstream regulation of terpene biosynthesis could alter the substrate geometry, raising the possibility of novel substrate isomers. This promiscuity with substrate geometry offers organism access to products, which are more cost-effective than evolutionarily expensive mutations. However, the product specificity of terpene synthases may depend on reaction conditions such as the concentrations of metal cofactors, pH level and temperature of the assays.
Multiple factors including some unknown factors govern the induction and regulation of terpenoid pathways [90,91]. Prenyldiphosphate synthase from the leaf beetle Phaedon cochleariae demonstrating a major switch in product specificity depending on the local concentration of a particular metal (Figure 7). P. cochleariae isoprenyl diphosphate synthase 1 (PcIDS1) enzyme, with metal cofactors Co2+ or Mn2+, produces 96% GDP and merely 4% FDP, whereas in the presence of Mg2+, PcIDS1 yields 18% GDP and 82% FDP.
A detailed study tested with in vitro PcIDS1 assays for the activity of allylic substrates (IDP, DMADP and GDP) and different divalent cations (Co2+, Mg2+, Mn2+, Ni2+ and Zn2+). The combined IDP and DMADP based assays with Co2+ as the metal cofactor resulted in maximum PcIDS1 activity. Assays with Co2+, PcIDS1 favorably condense IDP and DMADP to generate GDP critical for insect defense (Figure 8). In contrast, PcIDS1 with Mg2+ metal cation as a cofactor, prefers combination of cosubstrates IDP and GDP resulting in FDP, acting as a substrate for insect development [91]. The mode of action is currently investigated by crystal structure analysis. Farnesyl diphosphate synthase (FPPS) in Aedes aegypti (AaFPPS) expressed in the corpora allata shows a similar plasticity with different metal ions: Mg2+ ions lead to FDP formation, whereas Co2+ ions generate mostly GDP [92]. These examples may point to an interesting case of a local metal ion concentration, which regulate the metabolic pathways to which single enzymes can allocate their resources.
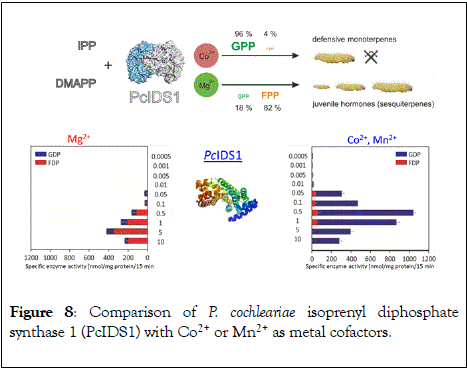
Figure 8: Comparison of P. cochleariae isoprenyl diphosphate synthase 1 (PcIDS1) with Co2+ or Mn2+ as metal cofactors.
Often metal cations such as Mg2+ and Mn2+ initiate the reaction cascade with the ionization of FDP by sesquiterpene synthases with binding of diphosphate moiety in the substrate [93-95]. However, also other metal ions are known for their catalytic efficiency in plant sesquiterpene synthases [95,96]. In case of the apple α-farnesene synthase, the activity is triggered by a monovalent potassium cation with a binding site positioned close to the entrance of the active site [97]. Δ6-protoilludene synthases from a fungus shows that divalent ions regulate cyclization specificity and product formation [98]. In terms of multiproduct synthases, a range of metal ions was tested for activity with TPS4 and TPS5 from maize and also MtTPS5 from Medicago truncatula (Figure 9). Variations in the product composition could be observed with each divalent metal cofactor [49,50].
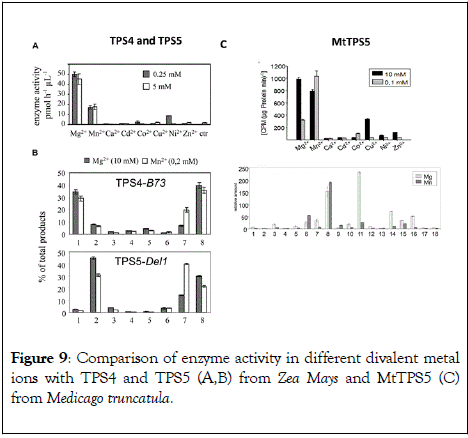
Figure 9: Comparison of enzyme activity in different divalent metal ions with TPS4 and TPS5 (A,B) from Zea Mays and MtTPS5 (C) from Medicago truncatula.
The product profile of Cop4 shifts dramatically to germacrene D with the substitution of Mg2+ by Mn2+ ions. The switch disfavors subsequent ring closures leading lead to the cadinyl intermediate and its tricyclic descendants. The Mn2+ ion due to its larger size has been suggested to reduce the space in the terpene synthase active site and thus, change the conformational flexibility of the bound substrate [99]. The product selectivity of the amorphadiene‐4,11‐diene synthase increased from 80 to 90 % in favor of amorphadiene in the presence of Mn2+ ions. The maize sesquiterpene synthases TPS6 and TPS11 and TPS4 and TPS5, increase premature quenching of carbocation intermediates upon replacement of Mg2+ with Mn2+ [52,95]. In TPS4 and TPS5, the maximum activity is observed with Mg2+ and Mn2+ ions. The MtTPS5 from Medicago truncatula, showed comparable maximum activity for both, Mg2+ and Mn2+ cations at different concentrations (Figure 9). However, with the switch in metal ions from Mg2+ cations to Mn2+ ions cubebol loses its major product status to germacrene D [49].
Multiproduct terpene synthases are most often membrane proteins, and the large number of products allows us to study the effect of pH level on individual product concentrations. An interesting shift was observed in MtTPS5 from Medicago truncatula: with increase in pH level, the product preference shifted from cadalane to germacrenes. At higher pH values, there was complete shift towards germacrene skeletons (Figure 10) [49].
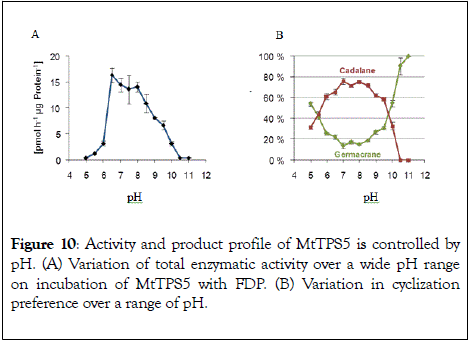
Figure 10: Activity and product profile of MtTPS5 is controlled by pH. (A) Variation of total enzymatic activity over a wide pH range on incubation of MtTPS5 with FDP. (B) Variation in cyclization preference over a range of pH.
In case of Cop4 and Cop6 from Coprinus cinereus, both multiproduct enzymes exhibit opposite behavior on pH variation. Cop6 demonstrates identical product profile under all conditions and 98 % of α-cuprenene from FDP. In case of Cop4, both alkaline and acidic conditions, it shows a preference for single major product down from three major compounds generated under neutral assay conditions. The cyclization cascade ends at pH 10 to produce (−)-germacrene D (91%) with the hydride shift and deprotonation of the germacradienyl cation, whereas at pH 5.0 cyclization continues besides (−)- germacrene D through a 1,6-ring closure to produce also a δ- cadinene (12 %).
Temperature is an environmental factor that usually affects reaction rates, but has also been reported to alter the profile of the multiproduct synthase Cop4 from Coprinus cinereus. Lowering the assay temperature from 25 to 4°C increased the selectivity of Cop4 from Coprinus cinereus for (−)‐germacrene D and reduced the fraction of structurally unidentified sesquiterpene olefins by ca. 50%. Further on, increasing the assay temperature from room temperature to 37°C, had the opposite effect and decreased the fidelity of Cop4. At room temperature Cop4 generated a relatively large fraction of products derived from the cadinyl cation intermediate namely β‐cubebene, sativene, δ‐cadinene and β‐copaene.
For immobile organisms like plants, multiproduct and multisubstrate terpene synthase provide a huge ecological advantage in the form of cost effective ways of adapting their chemical defensive repertoire. Unlike pheromone biosynthesis, in which product and substrate specificity of the terpenoid synthase is correlated with highly specific receptors in the receiving organism, plant defense uses its biosynthetic capacity to maximize the number and structure of compounds; in this way it can utilize multiple substrates and multiproduct enzymes as an adaptive mechanism for protection against a wider range of predators.
A direct impact of terpene volatiles for indirect defense is observed when maize in reaction to S. littoralis feeding releases terpene volatiles, these volatiles attract the parasitic wasp Cotesia marginiventris which after ovipositing eggs the egg of the herbivore S. littoralis causes its death (Figure 11). The attractive volatiles are generated by the multiproduct enzyme TPS10 with FDP, and include the major products (E)-β-farnesene, (E)-α- bergamotene, and additional sesquiterpenes [100]. Transgenic Arabidopsis plants overexpressing TPS10 enzymes in olfactometric analyses with the female parasitoid C. marginiventris and transgenic Arabidopsis plants demonstrated that the insect traced their host by associative learning of TPS10 derived sesquiterpenes. Overexpression of the TPS23 enzyme in Arabidopsis showed that the formation of (E)- β-caryophyllene is associated with damage of the plant by C. marginiventris [101]. In the soil (E)-β-caryophyllene is used for indirect defense by attracting entomopathogenic nematodes to the maize roots.
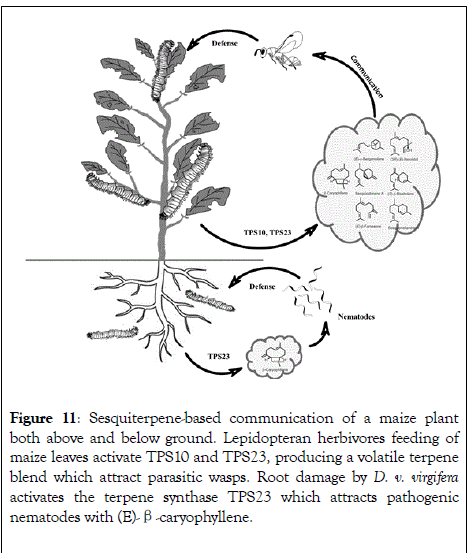
Figure 11: Sesquiterpene-based communication of a maize plant both above and below ground. Lepidopteran herbivores feeding of maize leaves activate TPS10 and TPS23, producing a volatile terpene blend which attract parasitic wasps. Root damage by D. v. virgifera activates the terpene synthase TPS23 which attracts pathogenic nematodes with (E)-β-caryophyllene.
On the other hand, the presence of multiple compounds and variations in individual components can be exploited by parasitic wasps like C. marginiventris, for an effective oviposition of eggs in different species of grass. These wasps showed recognition for different terpenoid blends to recognize diverse grass species during the course of field experiments to discern beneficial plants [102-104]. This strategy has been of benefit for agriculture by maize interplantation with Melinis minutiflora, reducing the number of stem borers by larval parasitism by Cotesia sesamiae [105]. Manipulation of terpenoid blends can be used as a possible strategy for pest management tool in crop plants by using indirect defense of plant. However, this requires comprehensive understanding of multiproduct terpene synthase pathways and their regulation factors in crop plants for engineering and regulation of desired volatile emissions [106].
In this chapter, we have shown examples of how multiple products and multiple substrates are used by multiproduct terpene synthases to enhance their product diversity and how these terpenoid volatiles can be easily modulated by assay conditions. Modulation of simple factors like pH level and metal cofactors and temperature can induce a dramatic shift in product profile. Significantly, isomers of substrates can lead to huge turnover advantage apart from the biosynthesis of novel product by using the alternative cyclizations cascade. This leads us to a hypothesis that variations in assay conditions can shift the enzymes native preferences, permitting the fast adaption of product profile using existing enzymatic architecture. This allows the organism to adapt its chemical arsenal against a predator avoiding costly genetic mutations spread over generations.
Citation: Vattekkatte A, Boland W (2020) Biosynthetic Origin of Complex Terpenoid Mixtures by Multiproduct Enzymes, Metal Cofactors, and Substrate Isomers. Nat Prod Chem Res. 8:372. DOI: 10.35248/2329-6836.20.8.372
Received: 28-Feb-2020 Published: 20-Mar-2020
Copyright: © 2020 Vattekkatte A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.