PMC/PubMed Indexed Articles
Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research - (2021) Volume 13, Issue 4
Bioequivalence Study of Favipiravir 200 mg Tablets in Healthy Thai Volunteers under Fasting Conditions
Ekawan Yoosakul1, Anas Sunhem1, Vipada Khaowroongrueng1*, Lalinthip Saeaue1, Busarat Karachot1, Isariya Techatanawat1, Porranee Puranajoti2 and Praphassorn Surawattanawan12International Bio Service Co., Ltd., 888 Golden Jubilee Medical Center, Mahidol University, Nakhon Pathom 73170, Thailand
Received: 22-Jun-2021 Published: 13-Jul-2021, DOI: 10.35248/0975-0851.21.13.436
Abstract
Favipiravir is a broad spectrum antiviral against RNA viruses. It has been considered as a promising treatment strategy for a pandemic of Coronavirus Disease 2019 (COVID-19). During this urgent need, the Government Pharmaceutical Organization (GPO), Thailand had developed favipiravir 200 mg tablet formulation (FAVIR®). A randomized, two-treatment, two-period, two-sequence, single-dose, crossover study was designed to determine the bioequivalence of two favipiravir 200 mg tablet formulations, FAVIR® and AVIGAN® under fasting conditions. The plasma-concentration time profiles were used to characterize the rate and extent of absorption of favipiravir in the test and reference products. The pharmacokinetics parameters were calculated using non-compartmental model. The analysis of variance did not show any significant difference between the two formulations. The 90% confidence intervals of geometric least squares mean ratio (test/reference) for log transformed parameters were within 80.00%-125.00% of bioequivalence criteria: 98.33%-108.31% for AUC0-tlast, 97.72%-106.89% for AUC0-∞ and 91.43%-112.32% for Cmax. Both products were well tolerated and no serious adverse events were reported. This study demonstrated bioequivalence between FAVIR® and AVIGAN® and supported interchangeable use between these products.
Keywords
Favipiravir; Bioequivalence; Pharmacokinetics; LC-MS/MS
Introduction
A pandemic of coronavirus disease 2019 (COVID-19) is associated with severe acute respiratory syndrome coronavirus-2 (SARS- CoV-2), a positive-sense single-stranded ribonucleic acid (RNA) virus [1]. More than 170 million confirmed cases and 3 million deaths have been reported worldwide since December 2019 and this public health crisis is highly possible to continue through 2021 [2]. To prevent more COVID-19 cases and deaths, efforts have been made to develop vaccines and therapeutic solution against SARS- CoV-2. As no specific treatments have been approved for treatment of COVID-19 so far, several existing antivirals have been considered for repurposing [3].
Favipiravir is a guanine analogue antiviral drug (Figure 1) which is converted to the active form, favipiravir ribofuranosyl-5’- monophosphate, by intracellular phosphoribosylation. Favipiravir demonstrates a broad spectrum of antiviral activities against RNA viruses by targeting RNA-dependent RNA polymerase to inhibit viral RNA synthesis [4,5]. It is effective against influenza viruses, and potentially inhibits the replication of other RNA virus including Ebola virus, flavivirus, chikun-gunya virus, norovirus and enterovirus [5]. Considering the mechanism of action and safety profile in human, favipiravir provides a promising treatment strategy for COVID-19. Several studies have demonstrated the efficacy of favipiravir through shorten vial clearance time, ameliorated clinical symptoms as well as improvement in chest imaging without significant safety concerns [6]. In Thailand’s treatment guideline for COVID-19, the use of favipiravir is recommended in a combination with Hydroxychloroquine and Protease inhibitors in mild cases with high-risk conditions (elderly population and patients with comorbidity) and pneumonia cases [7].
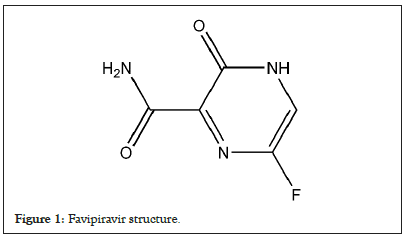
Figure 1: Favipiravir structure.
The recommended dosing regimen of favipiravir is 1800 mg twice a day on the first day, then 800 mg twice daily for the following 9 days (day 2-10). In response to this high demand during the COVID-19 pandemic, the Government Pharmaceutical Organization (GPO), Thailand had developed favipiravir 200 mg tablet formulation to comply with a mission to reserve the essential medicines and medical supplies for the country. The bioequivalence study was conducted to support the interchangeability between a generic product, FAVIR® and the reference product, AVIGAN®.
Materials and Methods
Study products
The test product used in this study was FAVIR®, favipiravir 200 mg tablets bearing lot no. S640014 manufactured by the Government Pharmaceutical Organization (GPO), Thailand. The reference product used in this study was AVIGAN® bearing lot no. HH2271 manufactured by FUJIFILM Toyama Chemical Co., Ltd., Toyama Factory, Japan.
Study subjects
The sample size was calculated by considering the maximum intra- subject variability for Cmax of favipiravir about 17.4%, T/R ratio at 95%, significance level at 5%, power at ≥ 95% and bioequivalence limits of 80.00%-125.00%. The calculation suggested that the sample size of 23 study subjects were sufficient to establish bioequivalence with adequate power. However, seven study subjects were added by estimating 20% dropouts and/or withdrawals.
Thirty study subjects were healthy Thai males and females at the age between 18 and 55 years with a body mass index between 18.0 and 30.0 kg/m2. They were estimated to be healthy by assessment of medical history, physical and laboratory examinations. All of them were well informed and gave voluntary written informed consent before participation in the study at International Bio Service Co., Ltd., Golden Jubilee Medical Center, Mahidol University, Thailand. Female subjects were not pregnant or breastfeeding at all time of the study and were instructed to use acceptable birth control methods (non-hormonal method) throughout the study.
Study subjects were not enrolled or withdrawn from the study if they met the exclusion criteria including history of hypersensitivity to favipiravir or any excipients of the study products, a recent history or presence of any disease, clinically significant illness within 4 weeks before the start of the study, any history of allergic reaction after taking any medications, alcohol dependence, drug abuse, cigarette smoking, recent any other clinical trial participation or recent blood donation within 3 months prior to the start of the study. Consumption of any medication, vitamins, dietary supplement, xanthine containing products or grapefruit/pomelo/orange-based products was restricted prior to dosing and during the study.
Study design
A randomized, two-treatment, two-period, two-sequence, single-dose, crossover study was designed to determine the bioequivalence of two favipiravir 200 mg tablets formulations under fasting conditions. The study was conducted following the study protocol which was reviewed and approved by the Institute for the Development of Human Research Protections, Thailand (COA no. IHRP2021034). Thirty study subjects were enrolled and randomly assigned to two groups, test-reference (TR) and reference- test (RT) according to the randomization schedule of receiving the product in each period of the study. After at least 10 hours of overnight fasting, a tablet of the test or reference product was orally administered to each subject with 240 mL of water, followed by mouth and hand check to assess the dosing compliance. The subjects were asked to remain in sitting or ambulatory posture for the first 1 hour after administration. After a washout period of 7 days, the procedure was repeated in the same manner to complete the crossover design. Adverse events were monitored throughout the study based on direct questioning, clinical examinations and laboratory examinations.
Blood sampling
A total of 19 blood samples were collected from each study subject in each period at pre-dose (0 hour), 0.083, 0.167, 0.25, 0.333, 0.5, 0.667, 0.833, 1, 1.25, 1.5, 1.75, 2, 2.5, 3, 4, 6, 12, 24 hours post- dose. Approximately 4 mL of blood at each time point was collected through an indwelling intravenous cannula in a forearm vein of the study subjects and transferred into vacutainers containing dipotassium ethylenediaminetetraacetate (K2EDTA) as an anticoagulant, and subsequently centrifuged at relative centrifugal force (rcf) 3000 ± 100 for 5 minutes at 4°C to separate plasma. Each plasma sample was divided into two separate aliquots for respective sample analysis, repeat analysis and incurred sample reanalysis. The plasma samples were stored upright at -55°C or colder until completion of analysis.
Study sample analysis and incurred sample reanalysis
The study samples were assayed using a validated liquid chromatography tandem mass spectrometry (LC-MS/MS) complying with the Principles of Good Laboratory Practice (GLP), European Medicines Agency (EMA) guideline on the investigation of bioequivalence [8], EMA guideline on bioanalytical method validation [9] and in-house Standard Operating Procedures (SOPs). The study samples of the same subject were determined for concentration in the same analytical run under the calibration range of 20.084-12973.313 ng/ml. Favipiravir and its internal standard, lamivudine were extracted from plasma using solid phase extraction method. Briefly, working solution of 100 ng/mL of lamivudine was added into plasma samples, followed by 0.1 N hydrochloric acid. Thereafter, the contents were loaded into the OASIS® MCX 1 cc/30 mg cartridges which were conditioned using methanol and 0.1 N hydrochloric acid. Then the cartridges were washed with water and dried under full pressure. Favipiravir and lamivudine were eluted from the cartridges using 5% v/v of ammonia in methanol. The eluents were evaporated at 50°C to dryness. The residuals were reconstituted with 70% v/v methanol and transferred into HPLC vials for subsequent analysis.
The sample analysis was performed on ACE® 5 C18 analytical column (4.6 × 100 mm) maintained at 40°C. An isocratic mobile phase consisting of methanol and 0.2 mM ammonium acetate (70:30, v/v) was delivered into Nexera™ LC system (Shimadzu Corporation, Japan) at a flow rate of 1 mL/minute. The detection was done using electrospray ionization triple quadrupole mass spectrometry (TSQ Quantum Ultra, Thermo Fisher Scientific Inc., USA) in the multiple reaction monitoring (MRM) transition of m/z 155.82 to 113.10 in negative ion mode for favipiravir and m/z 230.08 to 112.15 in positive ion mode for lamivudine. Data analysis was performed using Xcalibur™ 4.0.27.42 and LCquan™ 3.0.26.0 (Thermo Fisher Scientific Inc., USA).
According to EMA guideline on bioanalytical method validation [9], the study samples having concentrations close to maximum concentration and in the elimination phase of each subject in each period were chosen for incurred sample reanalysis in separate analytical runs. The concentration from incurred samples was not used for pharmacokinetic calculation.
Pharmacokinetic and statistical analysis
Non-compartmental model of Phoenix WinNonlin software version 6.4 (Pharsight Corporation, USA) was used to characterize the pharmacokinetics of favipiravir. The maximum plasma concentration (Cmax), area under the concentration-time curve from time zero to the last sampling time point (AUC0-tlast) and area under the concentration-time curve from time zero to infinity (AUC0- ∞) were considered as the primary pharmacokinetic parameters, whereas the time to reach Cmax (tmax), elimination rate constant (λ2) and half-life (t1/2) were considered as the secondary parameters.
The statistical analysis was carried out using PROC GLM of SAS® version 9.4 (SAS Institute Inc., USA). The analysis of variance (ANOVA) was used to determine the effects of the formulation, period, and sequence on log-transformed primary parameters (AUC0-tlast, AUC0-∞ and Cmax). The ANOVA model included sequence, formulation and period as fixed effects and subject nested within sequence as a random effect. Sequence effect was tested using subject nested within sequence as an error term. Two one-sided tests for bioequivalence and 90% confidence intervals (CIs) for the ratio of the geometric least squares mean (test/ reference) of log-transformed primary parameters were computed. Wilcoxon sign rank test was performed to compare the median tmax of the test and reference products. All statistical calculations were executed at a significance level of 5% (α=0.05).
Results
Demographic characteristics of study subjects
A total of 30 adult Thai male and female subjects were enrolled and randomly divided into TR and RT group equally. The mean ± SD of age, height, weight and BMI of enrolled subjects were 36.00 ± 9.62 years, 1.65 ± 0.08 m, 64.42 ± 9.77 kg and 23.55 ± 2.97 kg/m2, respectively. One subject was withdrawn by the principle investigator before check-in to period II since the subject was vaccinated against COVID-19 during washout period. Therefore, twenty-nine subjects completed the study and their plasma concentration data were used for pharmacokinetic and statistical analysis.
Study sample analysis and incurred sample reanalysis
All study samples including samples from withdrawn subject were completely analyzed. The samples from the same subject were analyzed in the same analytical run. The correlation coefficient of each analytical run calculated from 10 calibration standards was more than 0.98. The inter-day accuracy of 5 levels of quality control samples during the study ranged from 93.2% to 106.7% of their nominal concentrations. The coefficient of variation (CV) of quality control samples ranged from 6.1% to 7.2%. The study samples analysis was completed within 30 days from first blood samples collection which were within the established long-term stability of 92 days at -22 ± 5°C and -65 ± 10°C.
Incurred sample reanalysis was performed to ensure the reproducibility and reliability of the concentration data. According to the EMA guideline [9], a total of 118 samples were chosen for reanalysis and 99.2% of them had percent difference between the original and reanalyzed concentrations less than ± 20%.
Pharmacokinetic and statistical analysis
The pharmacokinetic parameters of the test and reference products calculated from 29 study subjects were summarized in Table 1. The mean plasma concentration-time profiles of the test and reference products were illustrated in Figure 2. From the results, the pharmacokinetics parameters of both products were comparable. Favipiravir was well absorbed with the maximum plasma concentration about 7800 ng/mL observed within 3 hours after administration for the test and reference products.
| Parameters | Mean±SD, N = 29 | |
|---|---|---|
| Test product | Reference product | |
| AUC0-tlast (ng.hr/mL) | 17990.369±6029.289 | 17385.099±5767.896 |
| AUC0-∞ (ng.hr/mL) | 18264.347±5999.958 | 17790.576±5651.616 |
| Cmax (ng/mL) | 7896.188±2505.382 | 7822.295±2815.117 |
| tmax (hr, in median (min,max)) | 0.5 (0.167,2) | 0.667 (0.167,3) |
| λ2 (1/hr) | 0.499±0.082 | 0.503±0.074 |
| t1/2 (hr) | 1.424±0.238 | 1.408±0.224 |
| Extrapolated AUC (%) | 1.725±1.511 | 2.686±1.926 |
Table 1: Pharmacokinetic parameters of test and reference products.
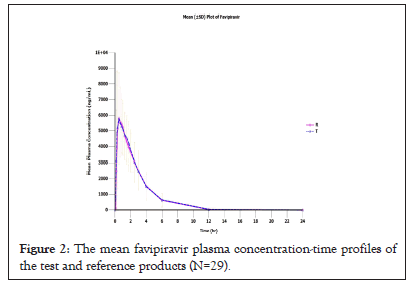
Figure 2: The mean favipiravir plasma concentration-time profiles of the test and reference products (N=29).
The data obtained from 29 study subjects who completed the entire study were used for statistical analysis and the results were represented in Table 2. The ANOVA showed no significant effects of period, formulation and sequence on log-transformed primary pharmacokinetic parameters (p>0.05). The 90% CIs for the ratio of the geometric least squares mean (test/reference) of log-transformed AUC0-tlast, AUC0-∞ and Cmax were within the bioequivalence criteria of 80.00%-125.00%. Wilcoxon sign rank test showed insignificant difference in median tmax between the two products (p>0.05).
| Parameters | Ratio (90% confidence interval) | Power (%) | Intra-subject CV (%) | ANOVA (p-value) | ||
|---|---|---|---|---|---|---|
| Period | Formulation | Sequence | ||||
| ln AUC0-tlast | 103.2 (98.33-108.31) | 100.0 | 10.8 | 0.3549 | 0.2769 | 0.5316 |
| ln AUC0-∞ | 102.2 (97.72-106.89) | 100.0 | 10.0 | 0.4137 | 0.4159 | 0.5624 |
| ln Cmax | 101.3 (91.43-112.32) | 97.2 | 23.3 | 0.1903 | 0.8275 | 0.4458 |
Table 2: Results of statistical comparison of primary parameters between test and reference products.
Tolerability
With concerning to the safety of study subjects, adverse events were closely monitored and recorded throughout the study. All adverse events were reported to the Institute for the Development of Human Research Protections, Thailand in a timely manner. A total of 20 adverse events were reported in 8 subjects after receiving the test product, while 17 adverse events were reported in 12 subjects after receiving the reference product as listed in Table 3. The mainly observed adverse events for both products were related to hematologic changes such as increase or decrease in basophil, hematocrit, hemoglobin, lymphocyte, monocyte, neutrophil, platelet count, red blood cell count and white blood cell count. However, all adverse events were mild in intensity and could resolve without any medical treatment. There was no serious adverse event reported in this study.
| Adverse events | Reported incidence (N) | |
|---|---|---|
| Test product | Reference product | |
| Decreased basophil | 2 | 2 |
| Increased hematocrit | 2 | 0 |
| Increased hemoglobin | 2 | 1 |
| Decreased/increased lymphocyte | 3 | 1 |
| Decreased/increased monocyte | 0 | 2 |
| Decreased/increased neutrophil | 2 | 0 |
| Increased platelet count | 1 | 0 |
| Increased red blood cell count | 0 | 1 |
| Increased total protein | 1 | 0 |
| Decreased white blood cell count | 0 | 1 |
| Increased glucose | 1 | 1 |
| Increased uric acid | 1 | 1 |
| Microscopic hematuria | 5 | 7 |
| Total | 20 | 17 |
Table 3: List of adverse events.
Discussion
Thirty study subjects were enrolled in the study but there was one subject was withdrawn by the principle investigator before checking- in to period II as the subject was vaccinated for COVID-19 and its effect on the pharmacokinetics of favipiravir has not been established. With twenty-nine study subjects who completed the study, the bioequivalence was established with the power of 100% for both AUCs and 97.2% for Cmax. Due to overestimating the dropout rate, it might lead to statistical overpower of the study.
The study was carried out under fasting conditions although food effect on Cmax has been reported. This effect becomes less significant at the higher dose or upon repeated dosing which can be explained by auto-inhibition of aldehyde oxidase, a key enzyme in primary metabolism of favipiravir [10]. Therefore, favipiravir can be taken with or without food. It is justifiable to conduct the study under fasting conditions considering that this is the most sensitive condition to detect formulation differences [8].
Pharmacokinetic parameters and profiles were comparable between the test and reference products as demonstrated in the concentration-time profiles of both products. The pharmacokinetics of favipiravir in this study was similar to the reported data in different ethnicities. The study in Japanese volunteers suggested that favipiravir was well absorbed after oral administration [11]. The study in Caucasian and Egyptian volunteers showed that the maximum plasma concentration were around 5000 ng/mL which were lower than that observed in this study even though the same dose was given [12,13]. At the study dose, half-life was approximately 1.4 hours which was comparable with reported half-life in Caucasian and Egyptian volunteers [12,13]. However, the reported half-life of favipiravir ranges from 2-5.5 hours after single dose administration. The increased elimination half-life is associated with the increased dose [14]. As evidenced by undetected concentration in any pre-dose samples of period II, seven days of washout period was sufficient for complete drug elimination.
Favipiravir was not considered as a highly variable drug since the intra-subject variability of primary pharmacokinetic parameters were less than 30% of the CV. From statistical comparison, there were no effects of period, treatment and sequence on the log- transformed primary pharmacokinetic parameters. The 90% CIs for the ratio of the geometric least squares mean of log-transformed AUC0-tlast, AUC0-∞ and Cmax were within the acceptance range. Moreover, Wilcoxon sign rank test showed no significant difference in median of tmax between two formulations. The results of this study indicated bioequivalence between the formulations in terms of rate and extent of absorption.
The adverse events observed in this study were in agreement with the published study data. The adverse events of favipiravir reported in Japanese with the study dose were relatively mild including increased uric acid, diarrhea and reduced neutrophil count [15]. It is important to note that the study dose was much lower than the recommended dose at which the adverse events should be monitored carefully. In addition, the tolerability for long-term use such as the potential for teratogenicity and QTc prolongation should be further investigated [16].
Conclusion
The statistical comparison of AUC0-tlast, AUC0-∞ and Cmax indicated that the two formulations of favipiravir, FAVIR® and AVIGAN® were bioequivalent. The test and reference formulations were well tolerated by the study subjects and no subjects developed serious adverse events. The bioequivalence study in healthy adult Thai volunteers under fasting conditions supported the use of FAVIR® as a generic substitute of AVIGAN®.
Acknowledgements
This study was supported by the Government Pharmaceutical Organization (GPO), Thailand.
REFERENCES
- Matheson NJ, Lehner PJ. How does SARS-CoV-2 cause COVID-19? Science (80- ) [Internet]. 2020;369(6503):510–1. Available from: https://science.sciencemag.org/content/369/6503/510
- Covid19.who.int. WHO Coronavirus (COVID-19) [Internet]. [cited 2021 Jun 10]. Available from: https://covid19.who.int/.)
- Dabbous HM, Abd-Elsalam S, El-Sayed MH, Sherief AF, Ebeid FFS, El Ghafar MSA, et al. Efficacy of favipiravir in COVID-19 treatment: a multi-center randomized study. Arch Virol. 2021 Mar;166(3):949–54.
- Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(7):449–63.
- Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Vol. 19, Nature reviews. Drug discovery. England; 2020. p. 149–50.
- Hashemian SM, Farhadi T, Velayati AA. A review on favipiravir: the properties, function, and usefulness to treat COVID-19. Expert Rev Anti Infect Ther. 2020;0(0):1–9.
- Department of disease control. Guidelines on clinical practice, diagnosis, treatment, and prevention of healthcare-associated infection for COVID-19 [Internet]. [cited 2021 Jun 10]. Available from: https://ddc.moph.go.th/viralpneumonia/eng/file/guidelines/g_CPG_06may21.pdf
- European Medicines Agency. Guideline on the Investigation of Bioequivalence. Committee for Medicinal Products for Human Use (CHMP), London. 2010.
- European Medicines Agency. Guideline on bioanalytical method validation. Committee for Medicinal Products for Human Use (CHMP), London. 2011.
- Lagocka R, Dziedziejko V, Klos P, Pawlik A. Favipiravir in Therapy of Viral Infections. J Clin Med. 2021 Jan;10(2).
- Du Y-X, Chen X-P. Favipiravir: Pharmacokinetics and Concerns About Clinical Trials for 2019-nCoV Infection. Clin Pharmacol Ther [Internet]. 2020;108(2):242–7. Available from: https://ascpt.onlinelibrary.wiley.com/doi/abs/10.1002/cpt.1844
- Morsy MI, Nouman EG, Abdallah YM, Zainelabdeen MA, Darwish MM, Hassan AY, et al. A novel LC-MS/MS method for determination of the potential antiviral candidate favipiravir for the emergency treatment of SARS-CoV-2 virus in human plasma: Application to a bioequivalence study in Egyptian human volunteers. J Pharm Biomed Anal [Internet]. 2021;199:114057. Available from: https://www.sciencedirect.com/science/article/pii/S0731708521001680
- Saglam O, Güney B, Saraner N, Sevici G, Dogan-Kurtoglu E, Ulusoy MG, et al. Bioequivalence study of two favipiravir tablet formulations in healthy male subjects. Int J Clin Pharmacol Ther [Internet]. 2021 Feb; Available from: https://doi.org/10.5414/CP203936
- Madelain V, Nguyen THT, Olivo A, de Lamballerie X, Guedj J, Taburet A-M, et al. Ebola Virus Infection: Review of the Pharmacokinetic and Pharmacodynamic Properties of Drugs Considered for Testing in Human Efficacy Trials. Clin Pharmacokinet [Internet]. 2016 Aug;55(8):907–23. Available from: https://pubmed.ncbi.nlm.nih.gov/26798032
- Agrawal U, Raju R, Udwadia ZF. Favipiravir: A new and emerging antiviral option in COVID-19. Med journal, Armed Forces India. 2020 Oct;76(4):370–6.
- Pilkington V, Pepperrell T, Hill A. A review of the safety of favipiravir - a potential treatment in the COVID-19 pandemic? Vol. 6, Journal of virus eradication. 2020. p. 45–51.
Citation: Yoosakul E, Sunhem A, Khaowroongrueng V, Saeaue L, Karachot B, Techatanawat I, et al. (2021) Bioequivalence Study of Favipiravir 200 mg Tablets in Healthy Thai Volunteers Under Fasting Conditions. J Bioequiv Availab. 13:436.
Copyright: © 2021 Yoosakul E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This study was supported by the Government Pharmaceutical Organization (GPO), Thailand.


