Research Article - (2021) Volume 9, Issue 1
Lessonia trabeculata, called “Aracanto”, is a brown macroalga distributed in submarine, exposed and semiexposed
rocky environments, and scattered along the Peruvian coast forming continuous grasslands. Despite its abundance in the Peruvian coastline, little or no importance has been given to its study, especially in the extraction of its secondary metabolites. In this work, we screened Lessonia trabeculata extracts for cytotoxic activity against human breast cancer cells. Only hexane extract was observed to be active against these cells. Both the chloroform and methanol extract were inactive. Bioassay-guided fractionation of the L. trabeculata hexane extract resulted in the isolation and identification of fucosterolusing NMR spectroscopy. The IC50 measured for hexane extract from L. trabeculata was 129 μg/mL and 75 μM for fucosterol, compared to mithramycin, a positive control. This study on the secondary metabolites of the Peruvian seaweed L. trabeculata is the first of its kind. The data obtained represents an interesting doorway for further studies.
Cytotoxicity, Fucosterol, Lessonia trabeculata, Macroalga, Seaweed
Marine organisms are considered as a source of active natural products when compared to plants and microorganisms. The rendering of these metabolites depends on collecting samples, isolation procedures, and spectroscopic techniques for identification. Cytotoxic and anticancer properties have been the main biological activity reported for natural marine products so far. The importance of secondary metabolites stemming from natural products in the drug discovery process has been well documented, with special attention to their role in medicine development [1].
Lessonia trabeculata is a brown macroalga, part of seagrass bedssubtidal strip known as “Aracanto”, forming forests and dense belts of important extension in intertidal and submarine environments in the central and southern Peruvian coast. The Lessonia trabeculata seaweeds are distributed along the east Pacific coast of the southern hemisphere, ranging from central Peru (12ºS) to Bahia Mansa-Orosno (40°S) in Chile [2]. However, despite having a vast marine and hydric biodiversity, there is no scientific research on the resources coming from seas and rivers as producers of essential metabolites, very useful in the nutritional, health and cosmetics aspects. In our country, most natural products are still mainly obtained from terrestrial plants. Peruvian marine algae have successfully been involved in many biological activities such as antibacterial, antioxidant, cardiovascular diseases, diabetes mellitus, and spermicide, among others [3]. Under this premise, marine algae are a potential source of compounds against cancer. At present, many available therapies have managed to increase the quality life and expectancy of cancer patients. Sadly, their use is often associated with side effects and therapeutic resistance. This fact demands continuous research of new bioactive metabolites.
In this context, marine products are a feasible anti-cancer alternative drug source, making it essential to investigate the vast marine biodiversity located in the coast south of Peru [4]. Consequently, this work aims at the isolation, and identification, of chemical constituents of L. trabeculata throughout a bioassayguided isolation to pinpoint anticancer compounds.
General Experimental Procedures
The 1H-NMR spectra were measured using AVANCE-400 NMR spectrometer (Bruker; 1H-NMR: 400 MHz) with CDCl3 as solvent. Column chromatography (CC) was carried out with Sephadex LH-20, and silica gel 60 (0.040–0.063 mm, Merck KGaA, Germany). Thin layer chromatography (TLC) was performed on a pre-coated silica gel 60 F254 (Aluminum sheet, Merck, Germany).
Collection of Algal Material
We collected the algal material from 2017 to 2018 at different points of the Peruvian south coast. Precisely, the area of study was comprised between the towns of Quilca and Matarani, in the Arequipa Region. We carried out eight sampling points for Lessonia trabeculata: El Faro (Quilca), El Inca, San José, Honoratos, Centeno, La Condenada, Mollendito and Catarindo. The sample was identified by Prof. Zevallos from IMARPE, Moquegua-Perú. A voucher Nº LT180717 is kept at the Natural Product Lab of the San Agustin National University of Arequipa, Arequipa, Peru.
Extraction and Bioassay-Guided Fractionation
The algae were cleaned and washed with fresh water. Then, dried and stored in plastic containers under refrigeration at -20°C until use. Our procedure involved the sequential extraction of the samples with organic solvents of increasing polarity. We took 300 g of the ground algae and then macerated them using the sequence of solvents: hexane (H; 750 mL), chloroform (CHCl3; 750 mL) and, finally, methanol (MeOH; 750 mL) for 72 h. The solvent/ alga mixture was separated by filtration, and the recovered tissue was subjected to the same process two times more. The three obtained solutions were combined, concentrated, and dried in a rotary evaporator at reduced pressure and at 40°C. Finally, three extracts were obtained: 10 g (H), 15 g (CHCl3) and 7 g (MeOH) respectively (Figure 1).
Figure 1: Structure of fucosterol isolated from the Macroalga marine Lessonia trabeculata.
Isolation and Bioassay-Guided Fractionation
The active hexane extract (9 g) of L. trabeculata was subjected to CC using hexane (100%; 3L), H-EtOAc (7:3; 3L), H-EtOAc (4:6; 3L), and EtOAc (100%; 3L) as the mobile phase giving four fractions I (3.5 g), II (1.5 g), III (2 g) and IV (1 g). Fraction II (1.2 g), which showed cytotoxic activity on MCF-7 cell line, was used for the purification process using CC by gravity and eluted with H-EtOAc (9:1; 5L), H-EtOAc (8:2; 5L) to yield fucosterol (0.7 g; IC50=75 μM) and a lipid fraction.
Fucosterol: 1H-NMR (400MHz, CDCl3) δH (ppm): 5.34 (1H, d, J=5.3 Hz, H-6), 5.17 (1H, q, J=6.6 Hz, H-28), 3.51 (1H, m, H-3), 1.57 (3H, d, J=6.7 Hz, H-29), 1.01 (3H, s, H-19), 0.99 (3H, d, J=6.5 Hz, H-21), 0.98 (3H, d, J=6.7 Hz, H-27), 0.97 (6H, d, J=6.9, H-26 and H-27), 0.69 (3H, s, H-18) [5]..
Cell Culture
The human breast cancer cell line MCF-7 (ATCC), derived from a pleural effusion of human breast adenocarcinoma, was seeded in 96-well plate (1 x 104 cells/well) using MEM cell culture media supplemented with 100 U/ml penicillin, 100 mg/ ml streptomycin, 2.5 mg/ml amphotericin B (all from Corning, Tewksbury, MA, USA), 10% fetal bovine serum (Hyclone, South Logan, Utah, U.S) and 10 mg/ml recombinant human insulin (GIBCO, Life Technologies, Grand Island, NY, USA). The normal counterpart MCF-10F was seeded (1 x 104 cells/well) in DMEM/F12 (1:1) supplemented with 100 U/ml penicillin, 100 mg/ml streptomycin, 2.5 mg/ml amphotericin B (all from GIBCO, Life Technologies, Grand Island, NY, USA), 10 mg/ ml recombinant human insulin (GIBCO), 5% equine serum (Hyclone, South Logan, Utah, USA), 0.5 mg/ml hydrocortisone (Sigma, St. Louis, MO USA) and 0.02 mg/ml epidermal growth factor (GIBCO, Life Technologies, Grand Island, NY, USA). The incubation conditions were established at 37˚C, complete humid atmosphere, 5% CO2 and 95% O2 [6].
Cytotoxic Assays
The cytotoxic effect of the extracts, fractions and purified compounds were assessed in MCF-7 cells in a dose-dependent manner. Cells were seeded for 1 day prior to exposure in a 96- well format in sextuplicate, reaching 70% confluence. After this incubation period, cells were exposed to concentrations of the extracts, or fractions ranging from 0 to 200 μg/ml (dissolved in 0.5% DMSO). The effect of each concentration was assayed for 24 h [6].
MTT Assay
Cell viability was assessed using the MTT assay [6]. 200 μl MTT medium was added to each well and incubated during 2.5 h at 37 °C and 5% CO2. An amount of 200 μl of DMSO was used for solubilization of formazan crystals. Absorbance was measured with a TECAN Infinite pro 200 plate reader at 560 nm. For each experiment, the culture medium was removed before exposure to eliminate serum, thus avoiding interference with the extracts and compounds. For background absorption, some wells remained cell-free with DMSO as blank control. The inhibitory concentration (IC50) was calculated using the 4-parameter logistic model (4PL) in GraphPad Prism 6.01 for Windows (GraphPad Software, San Diego, CA, USA). IC50 denotes the concentration of the compound that inhibits 50% of cell growth. Numerical data for viability was expressed as the mean ± standard error of the mean (SEM). A P<0.05 was considered to be statistically significant.
The dried seaweeds of L. trabeculata (0.3 kg) were sequentially extracted by maceration with hexane, chloroform and methanol at room temperature for three days. The filtered extracts were combined and evaporated under reduced pressure to obtain 10 g, 15 g and 7 g, respectively, of each extract. Then, the extracts were subjected to a bioassay on cytotoxic activity against the human breast cancer cell; with the hexane extract showing the best activity (Table 1). Finally, a combination of repeated CC on silica gel, along with bioassay-guided isolation, isolated fucosterol (Figure 2) and an unknown lipid fraction. Fucosterol was identified based on their NMR data [5] and resulted in low cytotoxic against human breast cancer cell (IC50=75 μM).
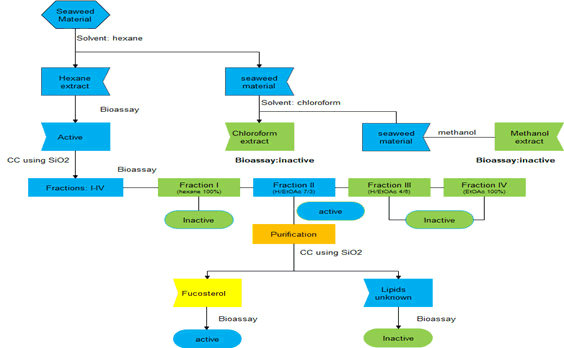
Figure 2: Process of bioassay-guided isolation from the hexane extract from L. trabeculata.
| Samples | Cytotoxicity on MCF-7 cell line |
|---|---|
| Control | - |
| Mithramycina | 6 ± 1.2 µM |
| Hexane extract | 129 ± 5 µg/mL |
| Chloroform extract | Not active |
| Methanol extract | Not active |
| Fraction I | Not active |
| Fraction II | 99 ± 15 µg/mL |
| Fraction III | Not active |
| Fraction IV | Not active |
| Fucosterol | 75± 5 µM |
| Fraction: Lipids | Not active |
Table 1: Cytotoxicity of extracts, fractions and fucosterol from L. trabeculata.
Phytosterols constitute a class of metabolites that are similar to cholesterol, both in structure and function. Phytosterols are grouped as sterols and stanols, and their biological activity is in their cholesterol-lowering potential. The contribution of phytosterols to human health is to hinder the intestinal absorption of cholesterol, thus lowering the risk factors of some chronic diseases, including cardiovascular disorders. Phytosterols possess numerous biological activities against cancer, diabetes, obesity, atherosclerosis, and Alzheimer’s, among many others. Seaweed are well-known to produce secondary metabolites including carotenoids, polyphenolics, polysaccharides, and sterols. Marine algae contain sterols like fucosterol in brown algae, cholesterol in red algae and β-sitosterol in green algae with similar properties to phytosterols in plants [4, 7].
Fucosterol has beneficial effects that include; anticancer, antioxidant, antidiabetic, anti-inflammatory, antibacterial, antifungicidal, antiulcerative actions; inhibition of cholesterol absorption; hepatoprotective, anti-phootoaging, anti-alzheimer disease properties, and it is also an angiotensin inhibitor [4]. In relation to anticancer activity, Tang et al. reported that fucosterol showed cytotoxicity against leukemia cells with IC50 values of 0.7 μg/mL [7-8]. Fucosterol, isolated from Sargassum angustifolium, exhibited cytotoxicity against breast and colon cancer lines with IC50 values of 27.94 and 70.41 μg/mL respectively [7, 9-10]. In addition, Fucosterol shows synergistic effect with 5-fluorouracil in 2D and 3D colon culture cell lines, without inducing cytotoxic in normal colon cells [11].
Furthermore, Jiang et al., 2018 demonstrated that fucosterol inhibits the PI3K/Akt/mTOR signaling pathway in the cervical cancer cell line. The PI3K/Akt/mTOR pathway is related to many cell processes such as proliferation, protein synthesis, survival, angiogenesis, apoptosis, and cell motility [12]. Mao et al., 2019 [13] reported that Fucosterol exhibited antiproliferative activity on human lung cancer cells by inducing apoptosis, cell cycle arrest and targeting of Raf/MEK/ERK signaling pathway (group of proteins that signaling from a receptor to the DNA). Another study by Caamal-Fuentes et al. reported that fucosterol, isolated from the hexane fraction of the seaweed Dictyota ciliolate, displayed antiproliferative effect by IC50=43.3 μg/mL (105 μM) on MCF-7 cancer cell lines [14]. In our case, cytotoxicity bioassayguided fractionations of the hexane extract were performed using MCF-7 cell line, yielded fucosterol. Although this compound is well known as a cytotoxic agent towards the different cancer cell lines showed above, our cytotoxic data for fucosterol against MCF- 7 cell line showed an IC50 value of 75 μM. This small difference could be attributed to external factors since the biological activity of a pure compound is independent from its source
Our bio-guided study on the cytotoxic metabolites from L. trabeculata against MCF-7 cell line yielded fucosterol with low cytotoxicity
To Instituto del Mar Peruano (IMARPE) Ilo headquarters, Perú.
This research was funded by INNOVATE-PERÚ, grant number 355-PNICP-PIBA-2014, Universidad Nacional de San Agustín de Arequipa Contract No. 07-2017-UNSA, and The APC was funded by FONDECYT REGULAR N° 1190314 (CHILE).
Received: 17-Dec-2020 Published: 07-Jan-2021, DOI: 10.35248/2329-6836.21.9.391
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.