PMC/PubMed Indexed Articles
Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2020) Volume 11, Issue 1
Azygos Vein Dissection during Pacemaker Electrode Lead Insertion: A Case Report
Suman Rai1, Zhe Jin2, Bing Wu1, Yang Xiang1, San-wu Wu1 and You-en Zhang1*2Postgraduate Training Base of Shiyan, Jinzhou Medical University, Shiyan 442000, China
Received: 25-May-2020 Published: 15-Jun-2020, DOI: 10.35248/2155-9627.20.11.345
Abstract
The invention of pacemakers has saved lives and improved the quality of life of countless patients. As the number of implantations has increased, so has the number of cases of post-implantation complications. To improve the ability to identify the complications of cardiac pacemaker insertion, it is necessary to remember that any type of intervention, however small, requires constant caution to achieve optimal surgical safety, minimal intraoperative risk, and individualized surgical methods, all of which can reduce the risk of complications of cardiac pacemaker insertion and maximize therapeutic benefits.
Keywords
Dissection; Pacemaker; Coronary angiography
Introduction
The invention of pacemakers has saved lives and improved the quality of life of countless patients. As the number of implantations has increased, so has the number of cases of postimplantation complications.Here we report a case of intraoperative azygos vein dissection and hemorrhage duringimplantation of a pacemaker electrode lead in the right atrium [1,2].
Case Report
A 46-year old woman was hospitalized due to depletion of pacemaker (VVI) battery. She had a medical history of sick sinus syndrome, paroxysmal atrial fibrillation, hypertension, and thyroid cancer. This patient was conscious and in active posture and she had no positive signs in the heart, lung, or abdomen upon physical examination. Results of the physical examination were as follows: heart rate, 60 beats per minute; blood pressure, 142/76 mmHg; respiratory rate, 20 beats per minute. The outpatient electrocardiogram showed sinus rhythm, third-degree atrioventricular block, and ventricular paced rhythm. Her program-controlled pacemaker had no obvious malfunction and the battery had >30,000 ohms resistance, indicating that it was due for replacement. Relevant examinations were completed during her hospitalization.
The color Doppler echocardiography showed left ventricular ejection fraction of 48% in M-mode echocardiography, 25% fractional shortening, >1 (E-wave/A-wave, mitral valve blood flow), left atrial diameter (anterior-to-posterior) of 41 mm, left ventricular diameter (anterior-to-posterior) of 51 mm, right atrial diameter of 41 mm (left-to-right), and right ventricular diameter (left-to-right) of 30 mm. No obvious abnormalities were detected in blood routine, kidney function, liver function, blood sugar, blood lipids, thyroid function, or tumor marker tests.
Coronary angiography (CAG) was carried out in supine position after routine disinfection of the right femur, right upper limb, bilateral neck, and front chest areas and surgical draping. Intradermal injection of 1% lidocaine around the puncture site of right iliac artery was given before placing the 6F arterial sheath, followed by administration of 3,000 units of heparin. The 5F Tig contrast catheter was sent to the root of the aorta along the guidewire. After withdrawing the guidewire, the end of the catheter was connected with three-way triple syringe to aspirate blood and remove the air bubble. Guided by contrast agent puffs, the catheter was placed into the left coronary artery for pressure measurement and multiposition angiography, followed by withdrawal of the catheter from the left coronary artery. The same procedure was repeated to place the catheter in the right coronary artery for pressure measurement and multiposition angiography.
The results showed right coronary artery dominance and no obvious stenosis in the left main coronary artery or the anterior descending coronary artery. No obvious stenosis was found in the left circumflex coronary artery or the right coronary artery. No collateral circulation was observed, the thrombolysis in myocardial infarction (TIMI) 3. We planned to upgrade the single-chamber pacemaker (VVI) to dual-chamber pacemaker (DDD). The right femoral venous puncture was performed after injection of 1% lidocaine for local anesthesia to insert a temporary pacemaker. After puncture of the left subclavian vein, multiple attempts were made to insert the guidewire, but they did not proceed smoothly. Subsequently, the contrast agent was injected through the puncture needle to create puffs in order to visualize the vessels, which showed curved and distorted left subclavian vein as well as plaques in the superior vena cava (Figure 1).
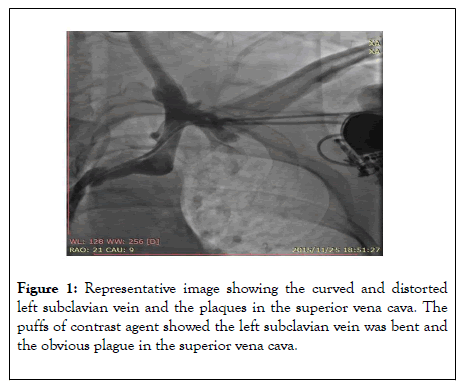
Figure 1: Representative image showing the curved and distorted left subclavian vein and the plaques in the superior vena cava. The puffs of contrast agent showed the left subclavian vein was bent and the obvious plague in the superior vena cava.
A tear-away venous sheath was sent along with an ultra-smooth loach guidewire, followed by removal of the sheath center and guidewire. The pacemaker electrode lead failed to reach the ideal position in the right atrium after many attempts. The patient complained of severe, pulsating pain in her right chest and back. The contrast agent was injected through the tear-away sheath and showed an azygos vein dissection and hemorrhage (Figure 2).
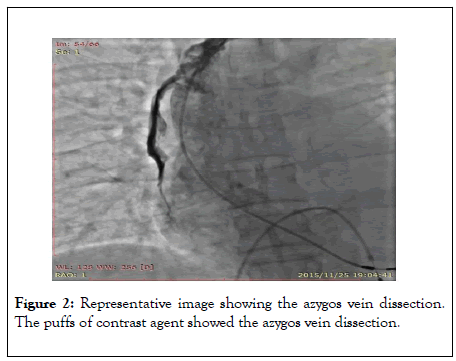
Figure 2: Representative image showing the azygos vein dissection. The puffs of contrast agent showed the azygos vein dissection.
The atrial pacemaker electrode lead was immediately withdrawn and replaced by the original VVI pacemaker generator. Tests showed that the pace-making and sensing functions of the pacemaker were good, so the operation was terminated. The patient was given intraoperative analgesia (3 mg bolus dose of morphine, twice), 10 mg bolus dose of protamine injection, blood pressure control (nitroglycerin injection), and symptomatic treatment. Bedside color Doppler sonography was carried out immediately and results indicated no pericardial effusion and no pleural effusion in the left thoracic cavity but mild pleural effusion in the right thoracic cavity. Symptoms were subsequently relieved, and vital signs were stable. After 30 minutes of monitoring, there was no readily visible increase in pleural effusion in the thoracic cavity and no pericardial effusion was observable through color Doppler sonography. The patient was then sent to coronary care unit for further intensive monitoring.
The patient received postoperative analgesia, blood pressure control, heart rate control, lipid regulation, diuresis, and symptomatic treatment. The color Doppler sonography performed 2 h after the operation showed no pericardial effusion, no pleural effusion in left thoracic cavity, but mild pleural effusion in right thoracic cavity. The color Doppler sonography performed on postoperative day 1 showed mild pleural effusion in the left thoracic cavity, mild-to-moderate pleural effusion in the right thoracic cavity, and no pericardial effusion. The computed tomography (CT) of the chest on postoperative day 3 showed bilateral pleural effusion and atelectasis in both sides of the lower lung (Figure 3).
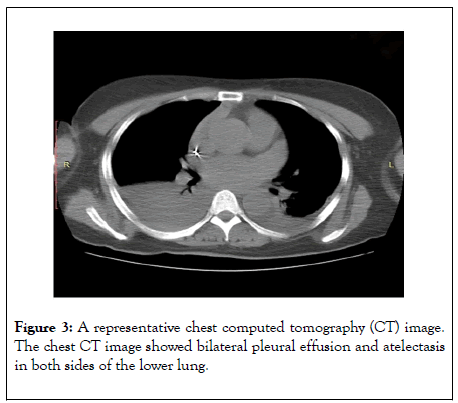
Figure 3: A representative chest computed tomography (CT) image. The chest CT image showed bilateral pleural effusion and atelectasis in both sides of the lower lung.
Dynamic monitoring of blood routine after the operation showed slightly decrease in hemoglobin in the patient. The color Doppler sonography on postoperative day 8 showed no pericardial effusion but mild pleural effusion in the right thoracic cavity. The patient recovered and had the surgical sutures removed on postoperative day 8. She was discharged on postoperative day 20. After 4 years of follow-up, the pacemaker sensing and pacing function of the patient remained in good condition.
Discussion
Insertion of the pacemaker electrode lead is a key part of pacemaker implantation. Guidewire-related complications mainly include pacemaker electrode lead displacement and breakage and vascular injury. It is generally believed that the azygos vein starts from the right ascending lumbar vein, arches around the root of right lung, and enters the superior vena cava approximately at the T4 level. There is a possibility of entering the azygos vein when placing the guidewireand electrode through a subclavian venous puncture because the probability of entering the azygos vein through the left subclavian venous puncture is higher than through the right side based on the anatomical structure Hemorrhaging can take place in the chest; it is prone to happen and challenging to terminate. If the hemorrhage is not treated in time, it might cause hemorrhagic shock and could endanger the patient’s life [3-6].
In this case, it appears that the pacemaker electrode lead was mistakenly inserted into the azygos vein, resulting in azygos vein dissection and hemorrhage, possibly due to the excessive force applied when moving the ultra-smooth loach guidewire and right atrial pacemaker electrode lead. The vital signs and hemodynamics of the patient were stable intraoperatively and postoperatively, so no thoracentesis was performed. This case is a reminder to use careful handling during the insertion of pacemaker lead to avoid endovascular damage caused by rough operation. The surgeons should use the patient's clinical response and fluoroscopy to determine the spatial relationship between the lead and the heart shadow, using contrast agent if necessary. For patients with prior pacemaker insertion, their vascular conditions should be fully evaluated before upgrading the pacemaker. For patients with vascular injury, especially those who had dissection and rupture, their vital signs and hemodynamics should be closely monitored, with surgical assistance if necessary.
Conclusion
Permanent implantation of cardiac pacemaker has been the most effective treatment for bradyarrhythmia, and various complications are the main factors affecting its therapeutic effect. To improve the ability to identify the complications of cardiac pacemaker insertion, it is necessary to remember that any type of intervention, however small, requires constant caution to achieve optimal surgical safety, minimal intraoperative risk, and individualized surgical methods, all of which can reduce the risk of complications of cardiac pacemaker insertion and maximize therapeutic benefits.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgment
This work was supported by the National Nature Science Foundation of China (no. 81500237) and Special Foundation for Knowledge Innovation of Hubei Province (nature science foundation) (No. 2017CFB563).
Author’s Contribution
Suman Rai and Zhe Jin contributed equally.
REFERENCES
- Eberhardt F, Bode F, Bonnemeier H, Boguschewski F, Schlei M, Peters W, et al. Long term complications in single and dual chamber pacing are influenced by surgical experience and patient morbidity. Heart. 2005;91(4):500-506.
- Madhavan M, Mulpuru SK, McLeod CJ. Advances and Future Directions in Cardiac Pacemakers. J Am CollCardiol. 2017;69(2): 189-210.
- Laohathai S. An isolated azygos vein rupture from blunt chest trauma. Asian CardiovascThorac Ann. 2019;27(9):757-759.
- Jiang LN. Common complications caused by pacemaker insertion and their treatments. J Electrocardioland Circulation. 2017;36(5): 309-311.
- Xueyin C, Yangang Su. The recognition and treatment of the pacemaker leads misplacing into azygos vein: 5 cases and literature review. J Cardiac Pacing and Electrophysiology. 2018;32(1):57-60.
- Drac P, Manak P, Klein J, Klein J, Kral V. Azygos vein injury in blunt chest trauma. Biomed Pap Med FacUnivPalacky Olomouc Czech Repub. 2007;151(2):347-348.
Citation: Rai S (2020) Azygos Vein Dissection during Pacemaker Electrode Lead Insertion: A Case Report. J Clin Res Bioeth. 10:345. doi: 10.35248/2155-9627.20.11.345.
Copyright: © 2020 Rai S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.