Citations : 1817
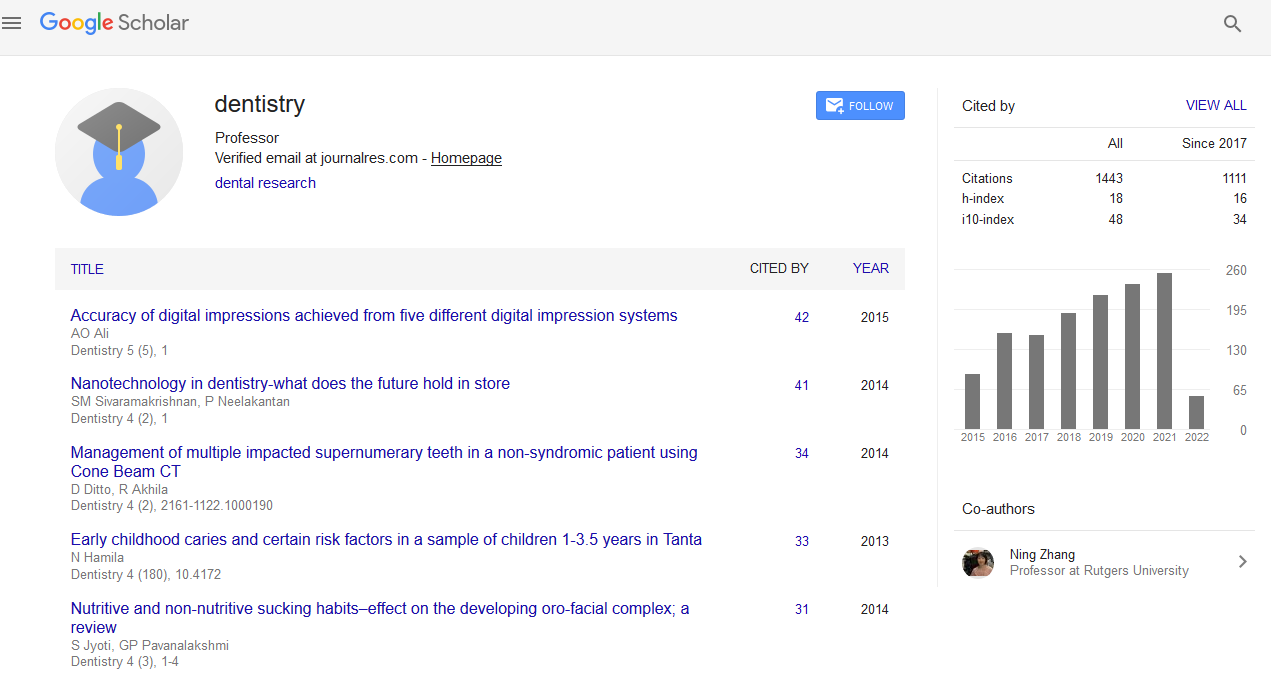
Dentistry received 1817 citations as per Google Scholar report
Indexed In
- Genamics JournalSeek
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- Directory of Abstract Indexing for Journals
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2019) Volume 9, Issue 6
Assessment of Bone Neoformation in Dental Alveolus with Platelet-Rich Plasma (PRP) in Rabbits (Oryctolagus cuniculus).
Tamae P.E.1, Bagnato V.S.2 and Panhóca H.V.3*2Institute of Physics of São Carlos-University of São Paulo (USP), São Carlos, São Paulo, Brazil
3Biophotonics Laboratory, Institute of Physics of São Carlos-University of São Paulo (USP), São Carlos, São Paulo, Brazil
Received: 19-Aug-2019 Published: 02-Sep-2019
Abstract
Objective: The purpose of this work was to assess the bone neoformation of a dental alveolus in PRP-grafted rabbits by means of a toluidine blue-stained experimental model using conventional light microscopy.
Material and Method: It was used thirty male rabbits of the New Zeland breed, divided into 5 control groups and 5 experimental groups. Each group was divided into subgroups to assess the postsurgery period after three, four, and eight weeks. Every rabbit was submitted to exodontia of the Right Lower Incisor (RLI) and its antagonist. Only the experimental group received PRP inside the RLI alveolus. The bone calcein-marker was ministered to every animal in the first and penultimate postsurgery week before sacrificing.
Results: Upon the analysis of the toluidine blue-stained blades, a cellular maturation was observed with intense osteogenic activity in the experimental group. When analyzing the fluorescence microscopy, it was verified that the bone neoformation presented a constant evolution in the 3, 4, and 8 week period in the control group. As to the experimental group, a quite significant peak was observed in the 4 week period compared to the control group p<0.05.
Conclusion: It was verified that the application of PRP propitiated a real acceleration of the bone neoformation.
Keywords
Growth factor; Bone neoformation; Platelets-rich plasma
Introduction
PRP is an organic atoxic non-immunoreactive matter which has been used to accelerate the paths towards cicatrization of surgical wounds both in soft and hard tissues [1,2]. Thus, PRP has been applied in several clinic situations, mainly in the othorhinolaryngology, neurosurgery, head and neck surgeries, and in the area of odontology [3], besides of plastic and reconstructive surgery, orthopedy, cardiovascular and pediatric surgery [4]. It is a product derived from the autogenous blood, rich in growth factors originated from the platelet-granules alpha [1], that allows a major concentration of a higher amount of platelets in small volume of plasma [2,5]. When PRP is added to a few white cells, it is granted a natural resistance to such product to infectious and/or allergic processes, improving the prognostic to the treatment [6,7]. There are several studies in the literature related to the attainment, benefits, and applicability of PRP, although very controversial that still must be duly analyzed and proved through experimental and clinical studies. The scope of this work was to assess the bone neoformation at the spot to the third medium of the dental alveolus in rabbits which received PRP graft, seeking to make quantitative and qualitative analysis under microscope of conventional and fluorescent light, occurred in different postsurgery periods.
Materials and Methods
It was used 30 young male rabbits of the New Zeland breed of the species Oryctolagus cuniculus with weights between 3.000 g to 4.000 g, Rabbits were divided into two groups: Group I (control group) was represented by Non-Platelet-Rich alveolus (NPRP) in which it was performed only exodontias, and Group II (experimental) that besides the exodontias, it was inserted PRP, represented by alveolus with Platelet-Rich Plasma (WPRP).
Obtaining the PRP
To attain PRP, it was followed the protocol performed in polypropylene, in order to avoid any damage to platelets along the cellular separation process. We performed a puncture of the auricular artery to collect a total of 10 ml blood [8].
Counting the platelets
After collecting the blood, the counting of the number of platelets present in the total blood was performed, using Newbauer chamber, according to the protocol adopted by the Hemocenter of the Irmandade Santa Casa de Misericórdia de São Paulo.
Obtaining the PRP
After counting the platelets, two centrifugations were performed: the first centrifugation at 200 rpm for 20 minutes following the protocol described [8]. As erythrocytes have higher density, they were settled down on the bottom of the tube. Above that layer, it was formed the buffy-coat containing several dispersive platelets [9]. Next, it was carefully transferred to the second tube, the supernatant plasma over 1 to 2 mm of the red series, that it contains higher platelets2, and then, it was submitted to the second centrifugation at 400 rpm for 10 minutes. After centrifugation, platelets were concentrated in a 1 ml plasma volume and then re-suspended. The counting of the number of platelets present in the PRP was performed in a 1:200 rate, that is, 10 μl of PRP in 200 μl ammonium oxalate at 1%.
Surgical procedure
After counting the platelets present in the PRP, the surgical procedure was initiated at the surgical rabbits' room of the anatomy Vivarium of the Instituto de Ciências Biomédicas da Universidade de São Paulo. As to the anesthesia, all animals received intramuscular injection of Acepromazine 2% (0,75 mg/Kg) by Univet with tranquilizer and pre-anesthetic; an intramuscular injection of Ketamine (35 mg/Kg) by Agener for general anesthesia, and an injection of dihidrotiazine (5 mg/Kg) by Bayer, in order to promote muscular relaxation, sedation and analgesic action. Once it was verified the absence of corneal reflex, the antisepsis was performed with 0.12% chlorhexidine gluconate. The infiltrating anesthesia was applied with a prilocaine hydrochloride solution with felipressin by Astra- Citanest, at the spot of the dental elements to be extracted, aiming to attain the vasoconstriction of the spot and later, a lower grade of bleeding and decrease in the postsurgery pain. The exodontia started with the Right Lower Incisor (RLI). Next, we performed the Bucco-dental-alveolus surgery maneuvers [10]. After the dental extraction, it was performed hemostasia of the dental alveolus using gauze, and the previously platelet gel was grafted. The platelet gel was performed in 1 ml PRP added to 0.2 ml thromboplastin, attained by the lyophilized extract of the rabbit’s brain (THROMBOMAX HS with calcium). The mucoperiosteal flap was duly separated in order to allow us to visualize the PRP being inserted in the alveolus; next, the flap was repositioned and sutured. After suturating the lower alveolus, we performed the Bucco-dental-alveolus surgical maneuvers 10 for the RUI exodontias (right upper incisor).
Preparing the marker solution
The calcein-marker powder was weighted in an analytical scale diluted in the powder form, weighted in an analytical scale and diluted in a physiologic serum and NA2HPO4 buffer solution [11]. It was subcutaneously ministered after the first postsurgery week, and in the penultimate week before sacrificing each animal.
Obtaining samples
All animals were sacrificed with a high dose of general anesthesia through a Ketamine endovenous ministered to attain the alveolus of the right lower incisor. Every alveolus sample from the right lower incisor was washed with watercourse for 5 seconds, and once numbered, they were fixed in a vessel containing 10% formalin for 7 days at room temperature. Samples were washed in a watercourse for 24 hours and dehydrated in a series of alcohols in the following concentrations: 60%, 70%, and 100%.
All samples were diaphanized in a xylol P.A. solution for 24 hours, and later for 48 hours. After the diaphanization, samples were submitted to imbibition and inclusion at the Multiuser Histology Lab chapel of the Instituto de Ciências Biomédicas da Universidade de São Paul–ICB III USP. For the imbibition, the numbered samples were arranged in a covered glass vessel containing “A” solution constituted by 15% of dibutyphftalate (Veteck-C14H10O4) and 85% of methylmethacrylate (Merck- C5H8O2) at room temperature for 4 days. After that period, samples were transferred to another covered glass vessel in “A” solution added by 1% of benzoyl peroxide-Merck, also remaining there for 4 days. Next, samples were individually arranged in hermetically sealed 40 ml glass vessels to perform the inclusion. Later, 5% of benzoyl peroxide was added to the “A” solution up to the 30ml mark and hermetically sealed.
After completing the polymerization, the cutting and polishing processes were performed. All samples were fixed in acrylic plate with cyanoacrylate ester and later, they were abraded in automatic grinding machine (EXAKT-Apparetebau, Nordestdt, Germain) using abrasive non-ferrous silicon carbide emery-paper with granulations: 800, 1000, 1.200, and polished in manual grinding machine (EXAKT 400CS Micro Grinding System) with emery-paper 2,400 and 4,000/mm² granulations.
Light microscopy (Toluidine blue): To check the cellular disposition of the bone neoformation in the different postsurgery periods, blades were toluidine blue-stained and observed and photographed in a photomicroscope NIKKONEclipse E600 under conventional light.
Fluorescence microscopy: The analysis of the calcein-marker was performed under fluorescence microscope coupled to a digital photograph machine by means of a 10X zoom objective. The amount of neoformed bone tissue in the different postsurgery periods of 3, 4, and 8 weeks was identified by the calcein-marker, and the computed area was selected and quantified by the software Image-Pro Plus.
Results
Analysis under light microscopy (toluidine blue)
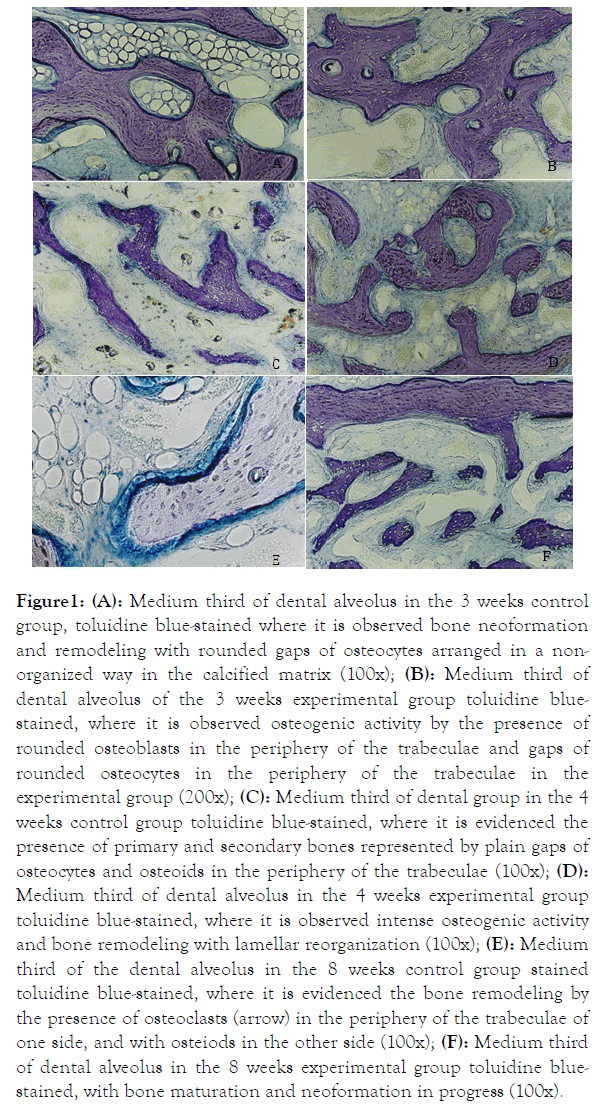
In the 3-week control group it was observed anostomosed bone trabeculae and primary and secondary bones in the same bone (Figure 1A).
Figure 1. (A): Medium third of dental alveolus in the 3 weeks control group, toluidine blue-stained where it is observed bone neoformation and remodeling with rounded gaps of osteocytes arranged in a nonorganized way in the calcified matrix (100x); (B): Medium third of dental alveolus of the 3 weeks experimental group toluidine bluestained, where it is observed osteogenic activity by the presence of rounded osteoblasts in the periphery of the trabeculae and gaps of rounded osteocytes in the periphery of the trabeculae in the experimental group (200x); (C): Medium third of dental group in the 4 weeks control group toluidine blue-stained, where it is evidenced the presence of primary and secondary bones represented by plain gaps of osteocytes and osteoids in the periphery of the trabeculae (100x); (D): Medium third of dental alveolus in the 4 weeks experimental group toluidine blue-stained, where it is observed intense osteogenic activity and bone remodeling with lamellar reorganization (100x); (E): Medium third of the dental alveolus in the 8 weeks control group stained toluidine blue-stained, where it is evidenced the bone remodeling by the presence of osteoclasts (arrow) in the periphery of the trabeculae of one side, and with osteiods in the other side (100x); (F): Medium third of dental alveolus in the 8 weeks experimental group toluidine bluestained, with bone maturation and neoformation in progress (100x).
In the experimental group, it was observed the presence of primary bone in osteogenic activity represented by rounded osteoblasts in the bone trabeculae periphery, osteoid located beneath the osteoblasts layer and gaps of rounded and disperse osteocytes in the calcified matrix (Figure 1B). In the 4 weeks controlling group, it was observed osteoid in the trabeculae periphery, gaps of osteocytes captive in the round and plain calcified matrix, clearly showing a tissue already in remodelation process, forming organized lamellas (Figure 1C). In the experimental group, we observed major trabeculae with intense osteogenic activity represented by gaps of the rounded osteocytes in the calcified matrix, arranged in a non-organized way and with lamellar disposition in the elongated trabeculae (Figure 1D). In the 8 weeks control group (Figure 1E), we observed a narrower trabeculate bone, presence of osteoids in the periphery of the trabeculae, and associated osteoclasts. In the experimental group, we observed the presence of primary and secondary bones in the same trabeculae, in an organized lamellar disposition and wider trabeculae.
Analysis under fluorescence microscopy
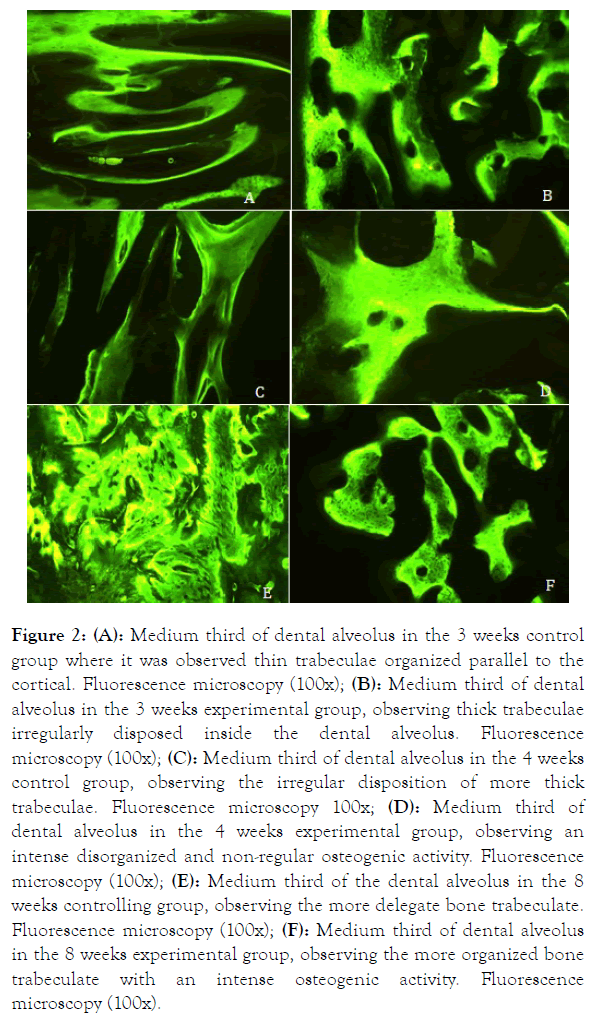
The presence of mineralized tissue was observed in every group and period. There was fine organized trabecula parallel to the alveolus cortical in the third medium portion of the 3 weeks control group (Figure 2A).
Figure 2. (A): Medium third of dental alveolus in the 3 weeks control group where it was observed thin trabeculae organized parallel to the cortical. Fluorescence microscopy (100x); (B): Medium third of dental alveolus in the 3 weeks experimental group, observing thick trabeculae irregularly disposed inside the dental alveolus. Fluorescence microscopy (100x); (C): Medium third of dental alveolus in the 4 weeks control group, observing the irregular disposition of more thick trabeculae. Fluorescence microscopy 100x; (D): Medium third of dental alveolus in the 4 weeks experimental group, observing an intense disorganized and non-regular osteogenic activity. Fluorescence microscopy (100x); (E): Medium third of the dental alveolus in the 8 weeks controlling group, observing the more delegate bone trabeculate. Fluorescence microscopy (100x); (F): Medium third of dental alveolus in the 8 weeks experimental group, observing the more organized bone trabeculate with an intense osteogenic activity. Fluorescence microscopy (100x).
In the experimental group, the trabeculae presented thickest and irregularly disposed into the alveolar space (Figure 2B). Along the 4 week period, an irregular disposition of the bone trabeculate with thicker walls was observed in the control group (Figure 2C); in the experimental group, the bone trabeculate arrangement was quite disorganized, but with an intense amount of mineralized tissue calcein-marked (Figure 2D). In the 8 weeks, it was observed in the controlling group the presence of thinner bone trabeculate (Figure 2E). In the experimental group, it was observed a more disperse bone trabeculate, but more thick with intense osteogenic activity, where it could be observed by the presence of round gaps disposed in a nonorganized way (Figure 2F).
Calcein markers applied in different periods were identified in the fluorescence microscope. This method allowed estimating the area in square the calcein. The calcein-marker applied in different periods was verified in the fluorescence microscopy. Such method allowed to estimate the area in square micrometers (μm²) in the medium third portion of the dental alveolus.
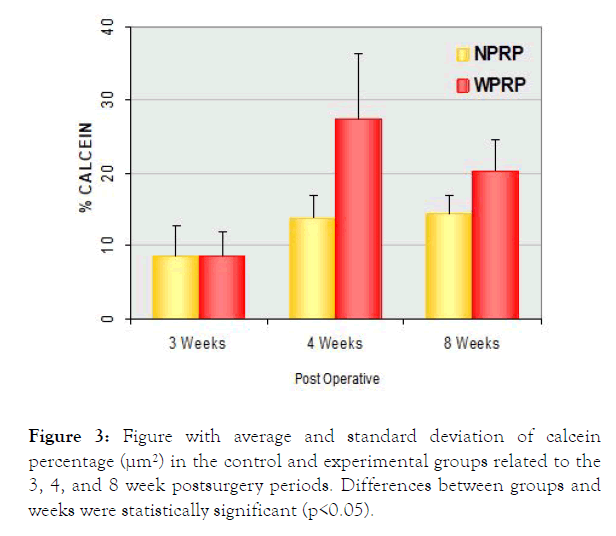
Chart and graphic 1 represent the average and standard deviation of the percentage of calcein in the control and experimental groups in 3, 4, and 8 postsurgery weeks (Figure 3).
Figure 3. Figure with average and standard deviation of calcein percentage (μm²) in the control and experimental groups related to the 3, 4, and 8 week postsurgery periods. Differences between groups and weeks were statistically significant (p<0.05).
Observation
It can be observed that the bone growth was similar in both groups. In the 4 weeks period there was a major bone neoformation in the experimental group than in the control group (p<0.05). In the 4 weeks period it was observed that in the experimental group there was a quite significant peak when compared to the control group, and in the 8 weeks period it was observed no significant difference between groups. In the experimental group there was a significant difference in the 3-4 weeks period and in the 3-8 weeks (p<0.05), but in the 4-8 weeks, such difference did not occur. On the other hand, when crossing the same period in the control group, results were constant (Tables 1 and 2).
| Animals | Platelets concentration | Platelets concentration in the RPR | Outcome |
|---|---|---|---|
| (weeks) | in the blood | in the RPR | (%) |
| 3 | 2,62,000 | 16,12,000 | 615.26 |
| 4 | 1,74,000 | 14,38,000 | 826.43 |
| 8 | 3,29,000 | 18,46,000 | 561.09 |
Table 1: Amount of platelets present in the total blood, in PRP and its outcome after the PRP concentration.
| Periods | Control | Experimental |
|---|---|---|
| (weeks) | (area-µm²) | (area-µm²) |
| 3 | 8.50 ± 4.11 | 8.58 ± 3.30 |
| 4 | 13.76 ± 3.23 | 27.34 ± 8.86 |
| 8 | 14.34 ± 2.58 | 20.35 ± 4.30 |
Table 2: Quantitative analysis. Average and standard deviation of the calcein area percentage (μm²) in the control and experimental groups. Differences between groups and weeks were statistically significant (p<0.05).
Statistical analysis
Data were submitted to the variance analysis (ANOVA) containing two factors (group and post-surgery weeks) followed by multiple comparisons using the Tukey method. The significance level adopted was p<0.05. The sine arch transformation of the square root of the calcein percentage (% calcein/100) was employed, aiming to homogenize variances.
Discussion
PRP is being used in surgical procedures bringing benefits, and at the same time, there are several on-going studies and trials to clarify the applicability. In order to attain an experimental model that can be standardized to perform future experiments, it was found in our work a favorable way to investigate PRP. Starting with the way to attain the blood, manipulation, counting of platelets present in total blood and PRP, as well as the best postsurgery period to confront future results and relevant factors to avoid degranulation of platelets and releasing of growth factors at an inconvenient moment.
Other relevant points to be considered are as to the ethylenediaminotetracetic acid (EDTA), as it is a nonrecommended anticoagulant to attain PRP since it evidenced samples with remarkable cellular destruction [12]. Blood collection must be performed before beginning the surgery, since the own surgery provokes the activation of the systemic coagulation mechanism, when there is an important amount of platelets present at the surgical spot, decreasing its concentration in the total blood [13].
Although there are several centrifugation equipments to promote PRP, the most important thing to be observed is the machinery specifications, such as radius of the centrifuge machine that will allow finding the number of rotations per minute. A very high speed might degranulate platelets inside the tube and releasing the growth factors inside it. Temperature is another important factor, and the optimum temperature is between 64 and 71.5°F.
We believe that one of the major factors that lead to a negative result as to the PRP applicability added to the postsurgery period analyzed and species studied is the way to attain and manipulate platelets, since they are quite sensitive, requiring very special care from the way to acquire them up to their application.
There are peaks of bone growth that vary from species to species. In rabbits, in the first 3 weeks of bone neoformation, there is no significant difference upon the use of PRP. In the study performed raising the maxillary sinus in humans, it was observed that there was no significant difference between the graft matter Bio-oss whether associated or not to PRP. The author mentions the importance to quantify platelets concentrated in PRP, since in the same plasma volume, it could be attained higher or lower platelets concentration when resuspended [14].
A 300% increase of the platelets concentration in the normal blood level [2]. In our study, we attained a lower concentration of more than 500%, and as much as 800% concentration, so verifying the need for more future studies related to PRP. Platelets, besides of promotion coagulation, also present other functions by the presence of the growth factors identified, facilitating the bone regeneration, improving the repairing of soft tissues, as it was observed in the study performed [1], in which they observed a significant increase in the thickness of the conjunctive tissue when associated to FCDP (platelet-derived growth factor) and FCI (growth factor similar to the insulin), as well as papers performed by Mustoe et al. and Fenis et al. who observed a major vascularization [15,16]. Similar fact was found in our experiment with the macroscopic analysis of every animal of the experimental group, where there was a quite fibrous thickening of the conjunctive tissue in the cervical portion of the dental alveolus where we performed the approach of the edges of the surgical wound; it was not found the same in animals from the control group. PRP improves the cicatrization of several tissues, such as the mucosa and skin [17].
We believe that platelets have several other functions than just hemostasia. Platelets presented several growth factors, and when they are secreted are responsible by the increase in the cellular mitosis, increase in the collagen production, recruitment of other cells that leads to damage, starting the vascularization and inducing the cellular differentiation [18].
Currently, new research on PRP applications has appeared in the literature with articles on PRGF® applications in other areas of dentistry. PRP has been applied to the Temporomandibular Joint (TMJ) for treatment of Temporomandibular Dysfunction (TMD) [19].
Experiments performed with intra-articular application of PRGF® in patients with TMJ osteoarthritis and limitation of mouth opening showed a significant decrease in joint pain and decrease in opening limitation. Another study applying PRGF® gel to dental fibrous hyperplasia wounds showed that this clinical application significantly improved hemostasis and epithelialization of the wound site. These studies show the possibility of developing clinical protocols enabling greater application of PRP in modern dentistry [19,20].
Conclusion
Thus, we verified that upon applying PRP, real bone neoformation acceleration is propitiated, and this may be applied in several areas of biomedical sciences, whenever the appropriate care is taken, such as the way to attain, store, manipulate, and centrifuge it, as well as its temperature and manipulation up to its application. PRP is capable to promote the faster bone neoformation, also increasing the amount of bone tissue, a faster and thicker trabecular formation, and increasing the cellular amount, promoting osteogenic activity to the formation and reabsorbing during the bone remodeling. New experiments with rabbits may be performed to attain PRP, investigating in a more specific way its constitution, properties, and functions.
Acknowledgment
CAPES, Financial institution that supported this project. The authors also acknowledge scientific contributions and helpful advice from Prof. Dr. Renato Paulo Chopard (in memory).
REFERENCES
- Lynch SE, Williams RC, Polson AM, Howell TH, REDDY MS, Zappa UE, et al. A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. J Clin Periodontol. 1989;16(8):545-548.
- Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma. Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):638-646.
- Whitman DH, Berry RL, Green DM. Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxill Sur. 1997;55(11):1294-1299.
- Buckley RC, Breazeale EE, Edmond JA, Brzezienski MA. A simple preparation of autologous fibrin glue for skin-graft fixation. Plast Reconstr. Surg. 1999;103(1):202-206.
- Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14(4):529-535.
- Lynch SE, Genco RJ, Marx RE. Tissue engineering: Applications in maxillo-facial surgery and periodontics. 2th ed. Carol Stream: Quintessence Books, 2007.
- Marx ER. Platelet-Rich plasma: A source of multiple autologous growth factors for bone grafts. In: Tissue engineering: Applications in maxillofacial surgery and periodontics. Lynch SE 2th ed. Carol Stream: Quintessence Books, 2007:1-82.
- Filho MAO. Platelet-rich plasma obtained in rabbits. Introduction of experimental model. ABCD. Arq Bras Cir Dig. 2008; 21(4):175-179.
- Givisyez FN, Pereirara JPM, Madalozzo TM, Marteleto MA. TELELAB. Ministério da Saúde-Coordenação Nacional de DST e Aids Coordenação de sangue e hemoderivados. 1998;21:80-92.
- Gregori C. Bucco-dental-alveolus. São Paulo em Perspectiva. 2004;18:92-102.
- König JB, Beck TJ, Kapper HF, Kapper CC, Masuko TS. A study of different calcification areas in newly formed bone 8 weeks after insertion of dental implants in rabbit tibias. Ann Anat. 1998;180(5):471-475.
- Landsberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58(3):297-301.
- Marx ER. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant Dentistry. 2001;10(4):225-228.
- Froum S, Wallace SS, Tamow DP, Cho SC. Effect of platelet-rich plasma on bone growth and osseointegration in human maxillary sinus grafts: three bilateral case reports. Int Periodontics Restorative Dent. 2002;22:45-53.
- Mustoe TA, Pierce GF, Morishima C, Devel TF. Growth factor-induced acceleration of tissue repair through direct and inductive activities in a rabbit dermal ulcer model. J Clin Invest. 1991;87(2):694-703.
- Fenis JPM, Stoelinga PJW, Jansen JA. Mandibular reconstruction: a histological and histomorphometric study on the use of autogenous scaffolds, particulate cortico-cancellous bone grafts and platelet rich plasma in goats. Int J Oral Maxillofac Surg. 2004;33(1): 48-55.
- Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
- Aghaloo TL, Moy PK, Freymiller EG. Investigation of platelet-rich plasma in rabbit cranial defects: a pilot study. J Oral Maxillofac Surg. 2002;60(10):1176-1181.
- Giacomello M, Giacomello A, Mortellaro C, Gallesio G, Mozzati M. Temporomandibular joint disorders treated with articular injection: the effectiveness of plasma rich in growth factors-Endoret. J Cran Surg. 2015;26 (3):709-713.
- Mozzati M, Mortellaro C, Gallesio G, Ruggiero T, Pol R. (2015). Surgical treatment of denture-induced fibrous hyperplasia with plasma rich in growth factors. J Cran Surg. 2015;26(3):772-775.
Citation: Tamae PE, Bagnato VS, Panhóca HV (2019) Assessment of Bone Neoformation in Dental Alveolus with Platelet-Rich Plasma (PRP) in Rabbits (Oryctolagus cuniculus) Bone Neoformation with Platelet-Rich Plasma. Dentistry 9:546. doi: 10.35248/2161-1122.19.9.546
Copyright: © 2019 Tamae PE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : CAPES, Financial institution that supported this project. The authors also acknowledge scientific contributions and helpful advice from Prof. Dr. Renato Paulo Chopard (in memory).