PMC/PubMed Indexed Articles
Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Original Article - (2019) Volume 10, Issue 1
Anti-Ovalbumin antibody production in mice following transdermal treatment
Greg Plunkett1*, Henry Legere2, Peter Hurwitz3 and James Strader42Austin Allergy Asthma and Immunology, Austin, Texas, USA
3Clarity Science, Austin, Texas, USA
4Endeavor Allergy Science, Austin, Texas, USA
Received: 14-Jan-2019 Published: 18-Feb-2019
Abstract
Background: Management of allergic rhinitis includes avoiding the exposure of allergens combined with drug therapy. For the patients that do not have substantial relief in allergic rhinitis symptoms or incur side effects, subcutaneous and sublingual immunotherapy can be a second line of treatment.
Objective: This study evaluated a topical route of immunotherapy administration of allergen through the skin which may be beneficial in treating allergic rhinitis. IgE, IgG and lymph node T cell responses in mice were studied using ovalbumin mixed with topical transdermal cream formulations containing a potential immune modulator applied
to the skin.
Methods: BALB/c mice were immunized with Ovalbumin (OVA) mixed with alum to induce an IgE response. After 14 days ovalbumin mixes with topical creams containing Toll-like receptor agonists were applied to the skin and IgE and IgG2a and were measured up to day 71. Results were compared with intraperitoneal injection controls. T-cell
biomarkers from draining lymph nodes and spleens were measured in mice treated with OVA creams after 3 days of treatment.
Results: Mice developed sIgE to ovalbumin after 14 days and application of cream formulations showed a dose dependent reduction in IgE compared to controls at 42 and 70 days. sIgG2 showed an increase in some cream formulation treated mice compared to intraperitoneal injection and biomarkers such as CD69 indicated that the
antigen reached draining lymph nodes.
Conclusions: Results from this mouse study demonstrated that topical administration of allergens mixed in creams can have an immune response and may provide an alternative route for immunotherapy.
Keywords
Allergy; Immunotherapy; Topical creams; Toll-like receptor agonists; Adjuvant; IgE; IgG2a
Introduction
Management of Allergic Rhinitis (AR) begins with avoiding the exposure of the allergens combined with drug therapy. Some of the common pharmacotherapies include antihistamines, intranasal glucocorticoids, or cromolyn mast cell stabilizers [1]. Despite providing some substantial relief in AR symptoms, these drugs are associated with side effects. One of the second lines of treatments is immunotherapy, which includes systemic injection of allergens in escalating, clinically tolerable doses [2]. Altering the mode of antigen delivery by mixing allergens with topical formulations may prove to be beneficial in treating allergic rhinitis in patients who have tried or failed subcutaneous immunotherapy and may confer substantially less side effects when compared to systemic medications.
Topical creams have been shown to transfer pharmacological compounds, such as ketamine, gabapentin, clonidine, and baclofen, through the skin to reach target organs [3,4]. The transdermal creams offer an advantage by reducing side effects such as upset stomach, and application in low blood flow areas and can offer higher doses with lower concentrations throughout the body.
In addition to providing the allergen to the target immune organs, such as subcutaneous and sublingual immunotherapy, the use of a topical cream can also add an advantage by addition of an immune modulator or adjuvant. Toll-like Receptor (TLR) agonists have been shown to enhance or suppress the activity of various cell types [5-7]. Some, such as TLR4 have been approved by the FDA. TLR7 agonist imiquimod in a 5% cream was approved in 1997 for a topical treatment of genital warts, keratosis, and basal cell carcinoma [8]. Toll-like receptor stimulus including TLR 7 are found to inhibit Th2 responses and therefore may have utility in treatment of allergic disease [9]. Therefore, it was hypothesized that the topical application of an allergen contained in a transdermal base cream would produce Anti-Ovalbumin (OVA) antibody production in mice. Inclusion of TLR agonist was studied to determine if potential immune modulation is possible, such as increases or decreases in the antibody response.
Materials and Methods
Mouse immunization study plan
All animal studies were conducted at Hooke Laboratories in accordance with IACUC guidelines using an approved protocol (1701PK-Tolerance). Nighty-six female BALB/c mice, age 9-12 weeks, were divided into 8 groups of 12 each. All mice received a single injection of 2 nanograms (ng) chicken Ovalbumin (OVA) mixed with alum intraperitoneally (i.p.) on day 0 in order to stimulate an immune IgG and IgE response. Over the next 12 days all mice were treated with 4 i.p. injections of 2 ng OVA/ alum every 3 days for a total of 5 injections.
On day 14 treatments continued according to the treatment schedule plan (Table 1). The test groups received 2 ng OVA diluted in Phosphate Buffered Saline (PBS); 1mL of this mixture was mixed with approximately 8 mL transdermal cream. The transdermal cream is a proprietary high molecular weight transdermal base cream that utilizes surfactants to transport the contents across the stratum corneum and is called the vehicle. The formulation is specifically designed for delivery of High Molecular Weight (HMW) proteins. The vehicle was applied to a shaved hair region of the back. Approximately 0.05mL of cream was applied to a 1-2cm2 area of the back skin at the same time of day (+/- 1 hr). Shaving anesthetized mice was done every 7 days and care was taken to not scratch the skin. Some of the test groups had a patent-pending proprietary formulation that included a Toll-Like Receptor (TLR) 7 agonist imiquimod (cream) at 3 concentrations added to the vehicle with OVA to assess immune modulating or adjuvant activity. The concentrations of imiquimod were 0.008%, 0.02% and 0.04%. Two groups were negative controls receiving either the vehicle cream mixed with PBS and delivered i.p. or just the vehicle added as a topical cream. Imiquimod was evaluated by comparing the immune response to the vehicle cream with the OVA and patent-pending formulations including TLR agonist containing creams at 3 concentrations as well as a group that contained the cream and imiquimod at 0.02% without additional OVA.
| Group | No. Mice | Treatment | Dose | Route | Frequency of Dosing | Purpose |
|---|---|---|---|---|---|---|
| 1 | 12 | PBS (Vehicle) | NA | i.p | Daily | Negative Control |
| 2 | 12 | OVA | 2ng | i.p. | Daily | Reference |
| 3 | 12 | Vehicle | NA | Topical | Daily | Negative Control |
| 4 | 12 | OVA | 2 ng | Topical | Daily | Test Group |
| 5 | 12 | T cell Adjuvant | 0.02% | Topical | Daily | Test Group |
| 6 | 12 | OVA+T cell Adjuvant | 0.01% | Topical | Daily | Test Group |
| 7 | 12 | OVA+T cell Adjuvant | 0.02% | Topical | Daily | Test Group |
| 8 | 12 | OVA+T cell Adjuvant | 0.04% | Topical | Daily | Test Group |
Table 1: Mouse immunotherapy anti-OVA experimental study group plan. PBS: Phosphate Buffered Saline; OVA: Ovalbumin; i.p: intraperitoneal injection, Vehicle, transdermal cream.
IgE and IgG2a immunoassays and T cell activation
Immunoglobulin E (IgE) and Immunoglobulin G2a (IgG2a) antibody production in vivo was measured using ELISAs for mouse IgE and IgG2a. Blood was drawn from mice and serum collected into Gel clot tubs for testing on day 14 then following specified treatment on days 42 and 70 days. Sera were stored at -80C until testing. Various dilutions of the serum were tested on OVA adsorbed plastic micro plates and followed ELISA kit instructions.
Three mice in 5 groups were studied for T-cell activation in spleen or lymph nodes from cream-treated mice. Ovalbumin specific CD4+ T cells were isolated from C.Cg-Tg (DO11.10) 10D lo/J mice and injected intravenously into recipient BALB/c mice. The transferred T-cells can be detected using the KJ1-26 antibody. Activation of these cells occurs when they encounter OVA. Activation was determined by analysis of expression of CD69 (early activation marker) on DO11.10 cells using flow cytometry. On Day 0, three C.Cg-Tg (DO11.10) 10 Dlo/J mice were humanely euthanized, and spleens and lymph nodes harvested. CD4+ cells isolated using a Dynal CD4+ isolation kit were obtained from a pooled cell suspension from all 3 mice for both spleens and lymph nodes. The isolated cell preparations were labeled with Carboxyfluorescein diacetate Succinimidyl Ester cell dye (CFSE) and injected into recipient BALB/c mice. The mice were assigned to 5 groups of 3 mice each and treated with PBS control (Group 1), OVA (Group 2) intravenously (i.v.), and a dose of 2 ng OVA in the proprietary base cream containing imiquimod (Groups 3-5). There was only a single treatment, on Day 1. On that day all recipient mice were anesthetized and hair was shaved from approximately 2 cm2 of the lower back. On day 2, Groups 1, 2 and 3 mice were euthanized and spleen and inguinal lymph nodes were collected. Upregulation of CD69 on CD4 cells specific for OVA and proliferation of CFSE cells were determined by flow cytometry. Lymph node and spleen cells were harvested from Group 4 on day 3 and Group 5 on day 4 and CD69 activation and CSFE proliferation were determined. A separate cell suspension was prepared for each tissue from each mouse (2 suspensions per mouse); cells counted and stained for flow cytometric analysis using CD4, KJ1-26 and anti-CD69 antibodies. The results are expressed as the proportion of CD69+ cells, as well as CFSEamong KJ1-26+CD4+ cells.
Statistical analysis
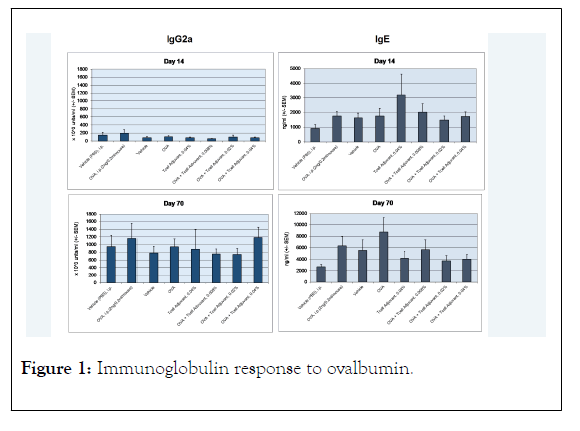
The mean was determined for the IgE and IgG2a data for each mouse group. The standard error of the mean was determined from the standard deviation of the individual mouse antibody values divided by the square root of the number of mice in the group and is represented by the error bars in the Figure 1. Significance of difference in the groups was done using Wilcoxon rank sum test, 2-sided using Astatsa program identifying p<0.05 and p<0.001 levels in Figure 1. The comparison of paired data of the treatment groups were with the OVA i.p. treated control.
Figure 1: Immunoglobulin response to ovalbumin.
Results
IgE and IgG2a immune response
Ninety-six female BALB/c mice were all treated with 2 ng OVA in alum i.p to stimulate an IgE and IgG response. The mice were divided into 8 groups of 12 mice each according to Table 1. There were 2 controls that received either PBS or OVA i.p. The other 6 groups received the cream vehicle with OVA and 0.008%, 0.02%, and 0.04% of the TLR agonist imiquimod. The initial analyses for IgE and IgG2a were performed on day 14 after 5 total i.p injections of OVA in alum. Following daily treatments with the control i.p. injections and cream formulations, blood was drawn from each group for analysis. The results for the initial time point and end of the study at day 70 are shown in Figure 1. At day 14, the IgG2a response was similar for each group with no statistical difference. After treatments with i.p. injections for controls and cream formulations the immune response increased more than 5-fold for each group. The highest imiquimod formulation with OVA mixed in cream was about equal to OVA injected i.p. and was higher than OVA only mixed in cream. There were no statistical significant differences between any of the groups. Increases in the OVA specific IgG2a also increased in the vehicle cream or PBS mice showing that the immune response continued up to day 70 despite ending OVA/alum injections at day 14. IgE responses were similar at day 14 without statistical significance in all groups; however, Group 5 with no OVA and 0.04% imiquimod had a higher mean specific OVA IgE response. At day 70, this group had reduced IgE and was significantly (p<0.05) different compared to the OVA i.p. control as the sIgE continued to increase in the OVA i.p. group. The imiquimod groups showed a dose dependent reduction in OVA specific IgE with a maximum of 78% in the 0.04% imiquimod group (p<0.001). The 0.008% group was significantly reduced to the OVA i.p. group (p<0.05) as well as the 0.02% group (p<0.001).
Mice treated intraperitoneally (i.p.) with 2 ng/mL OVA in alum were tested for mouse IgE and IgG2a. Treatment with OVA present in transdermal creams showed an increase in antigen IgG response similar to controls. There was a dose related decrease in OVA specific IgE in the TLR agonist (T cell adjuvant) treatment groups compared to the controls. *significance p<0.05, **significance p<0.001.
T-cell activation
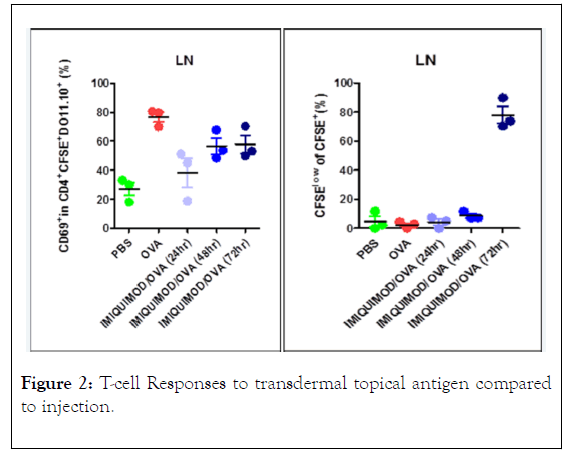
The results of the T-cell proliferation and activation experiments are shown in Figure 2. Three C.Cg-Tg (DO11.10) 10Dlo/J mice that were sensitized to OVA had both spleen and lymph node T cell isolated and a pool of these cells was injected into 5 groups of 3 BALB/c mice. Two groups of 3 mice each were injected i.v. once with either PBS or 2 ng OVA in PBS. Three groups of 3 mice were treated with 0.05 mL HMW cream with OVA and 0.04% imiquimod. All mice groups were anaesthetized and a 2 cm2 area shaved on the back to apply the cream OVA adjuvant immune modulator. After 1 day the spleen and lymph nodes were harvested from the PBS and OVA control mice and one group of the cream treated mice. Spleen and lymph nodes were isolated from 3 mice on day 3 and on day 4. Early activation of the T-cells with the treatments is detected with the CD69 marker and reported as a percentage of D011.101 T-cells transferred that can be identified with the K1-26 antibody and CFSE dye labeling. The CD69 activation was demonstrated in lymph nodes from the transferred cells in the OVA injected i.v. compared to the PBS control. The cream treatment showed an activation increase between the low PBS control and the OVA treated controls which increased 2 and 3 days after treatment suggesting slow release of the activating immune modulator. This large effect only occurred in lymph node cells and not in the spleen. Percentage of CFSE cells showing activation was shown only in the lymph nodes and only after 3 days after treatment. A small increase in the spleen was shown on day 2 and day 3.
Figure 2: T-cell Responses to transdermal topical antigen compared to injection.
Results from 3 mice in each group that were treated on day 1 with (OVA) then with either Phosphate Buffered Saline (PBS) or OVA and the proprietary transdermal topical cream with the TLR agonist adjuvant. CD69 and proliferation of CFSE cells were measured by flow cytometry.
Discussion and Conclusion
Alternative routes of administration of allergen compounds for immunotherapy can have potential advantages over subcutaneous injection or sublingual application of drops or tablets, including reduction of side effects, compliance or efficacy. Most immunotherapy methods performed in the US do not contain immune modulators with the exception of alum absorbed extracts that make up only a small percentage of treatment options. Alum can provide a slow release and possible adjuvant activity, however is still administered by subcutaneous injection.
In this study, using the egg ovalbumin model, a topical method was studied by delivering allergens to the skin using a proprietary transdermal cream mixture. This model uses mice that can be induced to produce specific IgE by injection i.p. small doses of protein. Once the mice are sensitized, changes in the amount of specific antibodies showed that further increases can occur over 14-70 days by continuing to inoculate the mice with ovalbumin. Even without injecting the antigen, specific IgG increases and IgE is maintained. This study showed that antigen contained in a specifically designed cream formulation and with added Toll-like receptor 7 agonist, had immune modulating activity. The IgG2a response was similar across all controls and tests with just a small non-significant increase at the highest concentration, 0.04% imiquimod. The biggest immune modulating effect was shown with IgE changes. IgE was shown to decrease with statistical significance from time of completion of sensitization injections in a dose dependent manner showing maximum effect at 70 days. This data along with the T-cell activation studies suggest that the ovalbumin can penetrate through the skin, interact with immune cells and cause a down regulation of IgE production.
In conclusion, topical application of an antigen present in a proprietary transdermal cream formulation to mouse skin resulted in a dose dependent decrease in IgE that was significant at a higher dose of TLR7 agonist imiquimod and a trend for increased IgG2 levels with increased doses. T-cell biomarkers demonstrated that the antigen was able to reach lymph nodes through the topical application of the proprietary formulation.
Initial results from this study provide evidence to suggest that topical administration of other allergens may be a potential method for allergen immunotherapy.
Acknowledgements
The mouse studies were carried out at Hooke Laboratories, Lawrence, MA
REFERENCES
- Cingi C, Kayabasoglu G, Nacar A. Update on the medical treatment of allergic rhinitis. Inflamm Allergy Drug Targets. 2009;8(2):96-103.
- Licari A, Ciprandi G, Marseglia A, Castagnoli R, Barberi S, Caimmi S, et al. Current recommendations and emerging options for the treatment of allergic rhinitis. Expert Rev Clin Immunol. 2014;10(10):1337-1347.
- Bassani A, Banov D. Evaluation of the percutaneous absorption of ketamine HCl, gabapentin, clonidine HCl, and baclofen, in compounded transdermal pain formulations, using the Franz finite dose model. Pain Med. 2016;17(2):230-238.
- Branvold A, Carvalho M. Pain management therapy: The benefits of compounded transdermal pain medication. J Gen Practice. 2014;2(6):188.
- Bhardwaj N, Gnjatic S, Sawhney NB. Toll-Like Receptor agonists: are they good adjuvants? Cancer J. 2010;16(4):382-391.
- Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Comm. 2009;388(4):621-625.
- Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27(3):370-383.
- Meyer T, Stockfleth E. Clinical investigations of Toll-like receptor agonists. Expert Opin Investig Drugs. 2008;17(7):1051-1065.
- Aumeunier A, Grela F, Ramadan A, Pham Van L, Bardel E, Alcala AG, et.al. Systemic Toll-like receptor stimulation suppresses experimental allergic asthma and autoimmune diabetes in NOD mice. PloSOne. 2010;5(7):e11484.
Citation: Plunkett G, Legere H, Hurwitz P, Strader J (2019) Anti-ovalbumin Antibody Production in Mice Following Transdermal Treatment. J Allergy Ther 10:1.
Copyright: © 2019 Plunkett G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.