PMC/PubMed Indexed Articles
Indexed In
- Open J Gate
- Academic Keys
- JournalTOCs
- ResearchBible
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2020) Volume 8, Issue 2
Antidepressant Treatment for Depression in Alzheimer’s Dementia: Systematic Review Article
Buciuta A, Vinasi RC and Coman HG*Received: 09-Jul-2020 Published: 27-Jul-2020, DOI: 10.35248/2329-8847.20.08.229
Abstract
Introduction: Alzheimer's Dementia (AD) is a neurodegenerative disease characterized by progressive cognitive decline. Neuropsychiatric symptoms (NPS) are frequently described in AD. Among the non-cognitive symptoms that can occur in AD, depression is the most common one.
Objective: The aim was to review published studies in order to examine the efficacy and safety of antidepressant medication for depression in AD.
Methods: An electronic database search was performed using the keywords: ” antidepressant ” , “ randomized controlled trial (RCT)”, “depression”, “dementia”, ”Alzheimer’s disease”. For the evaluation of efficacy, the reduction of scores on the evaluation scales of depression and the rate of response or remission were used and for the safety evaluation the rate of dropout and the rate of adverse effects were considered. The effect of antidepressant therapy on cognitive performances was also tracked.
Results: According to inclusion criteria, 14 RCT studies that aimed at assessing the efficacy and safety of an antidepressant in the treatment of AD associated depression have been selected, as well as 6 additional studies that use two or more antidepressants. The results show a debatable efficacy of these treatments. Among the included antidepressants, selective serotonin reuptake inhibitors (SSRIs) have proven to be most tolerated and without negative effects on cognition.
Conclusions: The available evidence does not provide sufficient support to uphold the efficacy and safety of antidepressant in the treatment of AD associated depression
Keywords
Antidepressant; Randomized controlled trial; Depression; Dementia; Alzheimer’s disease
Introduction
Alzheimer ’ s dementia (AD) is a chronic, debilitating, neurodegenerative disease characterized by progressive symptoms of cognitive and functional decline. Neuropsychiatric symptoms frequently occur over the evolution of the disease. There have been identified to accompany dementia, as well as AD, four groups of non-cognitive symptoms: depressive symptoms, psychotic symptoms, sleep impairment and behavioural disturbances. These symptoms can occur at any stage of the disease. Depressive symptoms are generally present shortly after the onset of AD, whereas symptoms such as behavioural disturbances can be noticed later on [1].
Depression diagnosed both as major or minor depressionrepresentsthe most common non-cognitive symptom in AD. The prevalence of depressive symptoms in different stages of AD ranges from 30% to 50% as seen in the majority of the studies [2].
Non-cognitive symptoms present in every form of dementia as well as in AD are associated with increased caregiver burden. More so, the management of these symptoms should also take into account the advanced age of the patients, making the therapeutic options more limited. Depression associated with dementia causes a further deterioration of cognitive functions which may be partially reversible if effective treatment is administered.
Treatment of depression associated to dementia includes pharmacological therapy, psychosocial interventions and electroconvulsive therapy. It has been shown the effectiveness of behavioural therapy in reducing depressive symptoms in patients with dementia [3]. ECT was shown to have positive effects in the treatment of dementia related depression, however with an increased risk of delirium superimposed on dementia [4].
The efficacy and safety of antidepressant medication in AD associated depression has been studied for over 30 years. Despite having an adequate design and being randomized, double-blind, placebo-controlled clinical trials, initial ones has a small sample size. Therefore their results are disputable and at times conflicting.
A wide range of antidepressants have been studied: cyclic antidepressants (tricyclic and tetracyclic), selective serotonin reuptake inhibitors (SSRIs), reversible inhibitors of monoamine oxidase A (RIMAs), serotonin-norepinephrine reuptake inhibitors (SNRIs), noradrenergic and specific serotonergic antidepressants (NaSSAs) as well as serotonin modulators and stimulators (SMS). Initially, studies have compared various antidepressants with placebo; subsequently other studies have approached the comparison of two or more antidepressants with or without placebo.
Once made possible, the results of the available studies have been summarized in multiple systematic reviews [5-9] as well as in several meta-analyses [8,10-14]. Even though progress has been noted, the current available results are not consistent enough to fully clarify the value of antidepressant medication when treating AD related depression. There are also instances when conclusions have been drawn as to the lack of efficacy of antidepressant treatment in dementia related depression [6].
The aim of the present study is to review randomized, doubleblind, placebo-controlled clinical trials in the available literature on the antidepressant treatment of AD related depression. The primary objective was to analyse the efficacy of antidepressants used for treating depression associated with dementia. The secondary objective is to evaluate the tolerability of these treatments as well as their effect on cognition.
Materials and Methods
Types of studies
Randomized, double-blind, placebo-controlled clinical trials that were conducted over a period of time of at least 4 weeks on single antidepressants were considered relevant. Studies that did not had neither placebo group, nor randomized allocation to groups, were not included. Studies that compared two or more antidepressants were also taken into consideration. Placebo group was not considered mandatory for the comparative studies, however double-blind randomized allocation to study groups was compulsory. The selection of the studies was based on more lenient inclusion criteria as to increase the range of available studies. Therefore, the sample size was not among the inclusion criteria.
Types of participants
In order to compare the results of the various studies, an acceptable degree of patients' homogeneity was considered. Therefore, the patients included in the studies has to met the diagnostic criteria for AD as per DSM-III-R – APA 1987, DSMIV – APA 1994, DSM-IV-TR – APA 2000, or ICD-9 – WHO 1979, ICD-10 – WHO 1999, as well as for coexisting depressive disorder diagnosed accordingly using the aforementioned guidelines.
Types of interventions
Inclusion criteria: Clinical trials targeting the administration of therapeutic doses of antidepressants in patients with dementia and associated depression were included. Concomitant administration of cholinesterase inhibitors and N-methyl-Daspartate (NMDA) receptor antagonists initiated over a period of time longer than 6 months prior to the inclusion in the studies, was permitted.
Exclusion criteria: Administration of central nervous system stimulants (amphetamines, methylphenidate, other amphetamine-like substances), mood stabilizers or other psychotropic medication (antipsychotics, benzodiazepines and other hypnotics) which is not considered to be antidepressant medication, represented an exclusion criterion.
Types of outcome measures
Primary outcomes: consited in evaluation of the efficacy of the antidepressive treatment. The criteria that were recorded as a measure of the effect of antidepressant treatment on the depression symptoms were the results obtained on the depression assessment scales: Hamilton Depression Rating Scale (HAM-D), Montgomery – Åsberg Depression Rating Scale (MADRS), Cornell Scale for Depression in Dementia (CSDD), as well as response rates (50% reduction of the initial depression score) together with remission rates (depression score drop beneath the considered threshold).
Secondary outcomes: consisted in evaluation of the tolerability of the antidepressive treatment. It was measured by the drop-out rate together with the presence and frequency of side-effects. The effect of pharmacological therapy on cognition was also considered an aspect of tolerability and was measured using validated clinical scales. Studies that have used Global Assessment Scales or Quality of Life Scales for dementia patients instead, were also included.
Electronic search
An electronic database search was performed on platforms such as Medline, Embase, Cinhal, Psychinfo, Cochrane Library, for the full text selection of articles that have been published by December 2019. The main keywords used were: “ Antidepressant ” , “ RCT ” (Randomized Controlled Trial), “Depression”, “Dementia”.
The additional search used terms for individual antidepressants (“imipramine”, “clomipramine”, “nortriptyline”, “desipramine”, “ maprotiline ” , “ mianserin ” , “ fluoxetine ” , “ citalopram ” , “ paroxetine ” , “ sertraline ” , “ venlafaxine ” , “ milnacipran ” , “mirtazapine”, “moclobemide”, ”vortioxetine”), or classes of antidepressants – (“tricyclic”, “related to tricyclic”, “SSRI”, “SNRI”, “RIMA”, “NaSSAs”, ”SMS”).
Data extraction and management
4311 articles have been identified using the keywords, respectively 3325 articles based on additional search. Duplicate papers have been excluded, the remaining ones adding up to 3044 articles. Next, the title and abstract of the papers were reviewed, excluding the ones that did not fit the objectives (2876). In order to evaluate the eligibility of the articles, the full text of 168 papers was reviewed. The homogeneity of the sample size and the tools used for the evaluation of the efficacy and tolerability of the antidepressants were taken into account. Finally, in the present study 24 articles have been included (Figure 1).
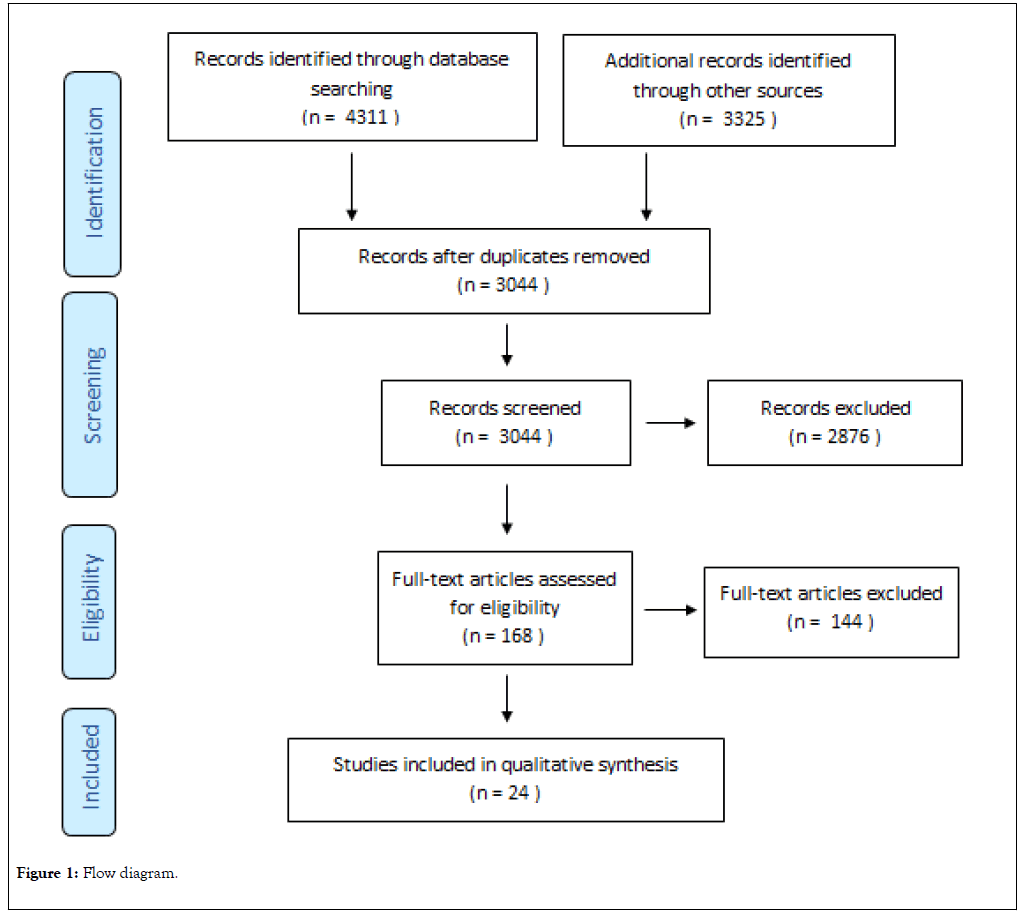
Figure 1: Flow diagram.
Results
The studies that met the inclusion criteria were identified and treated separately. Initially, studies that analysed the efficacy and tolerability of a single antidepressant compared to placebo in AD associated depression were considered. Subsequently, studies that compared two or more antidepressants with or without placebo were analysed.
Studies with a single antidepressant
According to the inclusion criteria, 14 studies have been selected to target the efficacy and tolerability of an antidepressant when treating AD associated depression. When data was available, the effect on cognition was also taken into account (Table 1).
| Sl no. | First author | Year | Antidepressant | Study design | Duration (weeks) | Sample size |
|---|---|---|---|---|---|---|
| 1 | Reifler BB | 1989 | Imipramine | RCT, Double-Blind | 8 | 61 |
| 2 | Nyth AL | 1990 | Citalopram | RCT, Double Blind | 4 | 98 |
| 3 | Fuchs A | 1993 | Maprotiline | RCT, Double-Blind | 8 | 127 |
| 4 | Petracca G | 1996 | Clomipramine | RCT, Double-Blind | 6 | 24 |
| 5 | Roth. M | 1996 | Moclobemide | RCT, Double-Blind | 6 | 694 |
| 6 | Magai C | 2000 | Sertraline | RCT, Double-Blind | 8 | 31 |
| 7 | Petracca G | 2001 | Fluoxetine | RCT, Double-Blind | 6 | 41 |
| 8 | de Vasconcelos UG | 2007 | Venlafaxine | RCT, Double-Blind | 6 | 31 |
| 9 | Lyketsos CG | 2003 | Sertraline | RCT, Double-Blind | 12 | 44 |
| 10 | Weintraub D | 2010 | Sertraline | RCT, Double-Blind | 24 | 131 |
| 11 | Rosenberg PB | 2010 | Sertraline | RCT, Double-Blind | 24 | 131 |
| 12 | Drye LT | 2011 | Setraline | RCT, Double-Blind | 12 | 131 |
| 13 | Choe YM | 2015 | Escitalopram | RCT, Double-Blind | 52 | 74 |
| 14 | An H | 2017 | Escitalopram | RCT, Double-Blind | 12 | 84 |
Table 1: Single antidepressant studies compared to placebo.
The first study that met the inclusion criteria, compared imipramineto placebo in the treatment of AD related depression. The study was carried out over a period of 8 weeks and included 61 patients, 28 of which had been diagnosed with dementia and depression, respectively 33 who have been diagnosed with dementia only. Assessment tools that have been used comprise of: HAM-D – to quantify the severity of depression, Mini-Mental State Examination (MMSE) and Dementia Rating Scale (DRS) – to quantify the cognitive impairment as well as the impact of the treatment on cognition. Patients with both dementia and depression did not indicate significant differences in the treatment response in terms of the depression severity between the imipramine and placebo subgroups. MMSE scores indicate a slight improvement of the cognitive performances in patients with dementia and depression treated with imipramine compared to placebo subgroup, without having significant differences. The DRS scores of the patients with dementia, both with or without associated depression who underwent treatment with imipramine had significantly reduced scores compared to placebo subgroup. The tolerability – which has been evaluated via drop-out rate did not reveal significant differences between neither of the described groups [15].
In order to assess the efficacy and tolerability of citalopram in treatment of emotional disturbances in patients with dementia, Nyth et al. conducted a study involving 98 patients with dementia. For the evaluation of treatment efficacy, the following assessment tools have been used at baseline as well as after 4 weeks of treatment with citalopram or placebo: Gottfries-Bråne- Steen Scale (which consists of subscales measuring intellectual, emotional and activities of daily living), and MADRS. After 4 weeks of treatment with citalopram, MADRS scores have been significantly improved compared to the placebo group. There was no change in the motor or the cognitive functions in neither group. The global assessment of side-effects did not show significant differences between the two groups and the drop-out rate was comparable [16].
A clinical trial conducted on an 8-week period including a sample size of 127 patients with depression associated to dementia, have described the administration of maprotiline to have significant improvement regarding depression symptoms assessed using the Geriatric Depression Scale (GDS) compared to the placebo group. Cognitive performance, assessed using MMSE, was improved in the placebo group, whereas in the study group cognition was not influenced whatsoever. Within the maprotiline group the drop-out rate was higher compared to the placebo group as an indicator of low tolerability despite the ratio of side-effects not being significantly different between the two groups [17].
For the assessment of the efficacy and tolerability of clomipramine, a 6-week study including 24 patients with dementia related depression was conducted. The patients from the 2 groups – the first one treated with clomipramine and the second one with placebo – were evaluated at baseline, two and four weeks, respectively at the very end of the study. HAM-D was used as an indicator for depression and MMSE for the assessment of cognitive performance. Clomipramine has been shown to be effective in improving depression even at the 2-week endpoint. There was a significant reduction in the HAM-D score compared to the placebo group at week 2, 4, as well as the 6th week, with even higher rates of remission at week 6. However, cognitive performance decreased in the clomipramine group, whereas in the control group they have remained constant. When it comes to tolerability, both drop-out rate and side-effects were significantly increased in the study group compared to the control group [18].
A 6-week study conducted on 694 elderly patients with cognitive decline and depression, aimed to assess the efficacy and safety profile of the treatment with moclobemide. As indicators of the effect of moclobemide on depressive symptoms, HAM-D and GDS were used. To evaluate the effect on cognition, MMSE and Sandoz Clinical Assessment-Geriatric scale (SCAG) were applied. Moclobemide had a significant effect over depressive symptoms compared to placebo, supported by the decrease in scores for both assessment tools used for depression. This conclusion was also supported by the higher response rate in patients within the moclobemide group. In terms of the cognitive performance, the study group had improved MMSE scores, however not statistically significant compared to the control group. The tolerability, assessed via the drop-out rate and side-effects did not differ between the two groups [19].
Another 4-week study with a sample size of 31 patients with AD associated depression conducted over the course of 4 weeks compared the efficacy and tolerability of sertraline and its effect on global functioning to placebo. Depression was evaluated with CSDD and Gestalt Scale (GS), whereas the global functioning was assessed with Cohen-Mansfield Agitation Inventory (CMAI) and Aversive Behaviour Feeding Scale (ABFS). For all aforementioned assessment tools there has been a tendency for improvement for the group treated with sertaline, however, without any statistically significant results being highlighted. There was no difference in the drop-out rate described for neither group [20].
The study published in 2001 by Petracca et al. ran over the course of 6 weeks, including 41 patients, evaluated the efficacy and tolerability of fluoxetine in the treatment of depressive symptoms for patients with dementia. Patients’ evaluation was more complex, including: SCID-I for diagnosis confirmation, HAM-D to evaluate the severity of the depressive symptoms, Clinical Global Impression (CGI) Scale for the global assessment of the patients ’ status, Hamilton Anxiety Rating Scale (HAM-A) to evaluate the anxiety intensity. Despite HAMD and HAM-A having improved after 6 weeks for both groups, no significant differences have been noted. Remission rates were identical in both groups. In neither of groups was the cognitive performance influenced. There were no statistically significant differences in terms of drop-out rate or side-effects in the two groups [21].
The efficacy and safety profile of venlafaxine in treating major depression associated with dementia were studied over a period of 6 week including 31 patients. To quantify efficacy, MADRS and the Clinical Global Impression-Severity (CGI-S) Scale were used. Tolerability was quantified via drop-out rate and the registration of side-effects. MADRS and CGI-S scores presented a modest decrease, without significant differences between the two groups. The side-effects did not reveal differences between the study and control group and drop-out rate was higher in the venlafaxine group compared to placebo [22].
The study published by Lyketsos et al. conducted on the course of 12 weeks and included 44 patients evaluated the safety and efficacy or sertraline in Alzheimer ’ s Disease patients and associated depression used as efficacy indicators the Cornell Scale for Depression in Dementia, Hamilton Depression Rating Scale, Psychogeriatric Depression Rating and Neuropsychiatric Inventory. The safety evaluation focused on the potential cognitive negative side effects, evaluated through the Mini- Mental State Examination and the potential decline in daily functionality evaluated with the use of the Activities of Daily Living Scale. Sertraline has been shown to be superior to placebo in the treatment of depression associated with Alzheimer’s disease. Also, a favourable influence was observed regarding the slowness of thinking, behavioural disorders and functional status, without altering the cognitive status [23].
Sertraline ’ s efficacy and safety profile were evaluated over a period of 24 weeks with a sample size of 131 patients with AD and associated depressive symptoms. Patients were evaluated at baseline, at 12 and 24 weeks using: Alzheimer ’ s Disease Cooperative Study Clinical Global Impression of Change index (mADCS-CGIC) and CSDD for the treatment effect on the severity of the depressive symptoms, MMSE for cognitive performance, the Neuropsychiatric Inventory (NPI) for the evaluation of the remaining non-cognitive symptoms that can be associated with AD, ACDS-Activities of Daily Living Scale (ACDS-ADL) to evaluate functionality of the patients, and The Alzheimer’s Disease-Related Quality of Life (ADRQL) Scale to assess life quality amongst the patients. Regarding the efficacy of sertraline treatment, there were no notable improvements of mADCS-CGIC and CSDD scores, as well as in response-rate and remission. No significant differences between groups were recorded for neither of the assessment tools applied. Drop-out rate and side-effects had similar results, being non-significantly different between the study and control group. One notable aspect was the higher rate of severe side-effects present in the sertraline group compared to placebo [24].
Rosenberg et al., present a study over a 12-week period of time, including 131 patients, assessing the effectiveness and tolerability of sertraline. The study used the same patients and inclusion criteria as those in the previously presented one. Despite being less consistent in terms of assessment tools used compared to Weintraub et al., the results presented are similar [25].
The double-blind RCT conducted by Drye et al. used as primary indicators for the evaluation of the depression associated with Alzheimer ’ s Disease the modified Alzheimer's Disease Cooperative Study Clinical Global Impression of Change (mADCS-CGIC). The study was carried over a period of 12 weeks and included 131 patients. No improvements in major depression (MaD), minor depression (MiD) or Alzheimer's Associated Affective Disorder (AAAD) were observed [26].
Choe et al., published in 2015 a study that aimed to evaluate the effect of escitalopram on the cognitive function of patients with AD as well as the antidepressant effect over the associated depression. In order to assess the effects of escitalopram over the cognitive decline, the team followed the changes rates of hippocampal and whole brain volume on magnetic resonance imaging and used the following scales: Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-cog) and MMSE. The Neuropsychiatric Inventory was applied in order to assess the associated psychiatric symptoms, whereas for the assessment of depression CSDD was applied. No significant changes have been shown between the two groups in terms of the changes of hippocampal or whole brain volume. On the other hand, escitalopram has proven to have significantly favourable effects over placebo when comparing CSDD scores at 28 weeks. However, these results were not consistent until the end of the study [27].
An et al., RCT for evaluating the efficacy and tolerability of escitalopram over the course of 12 weeks included 84 patients with AD and associated depression, diagnosed according to Olin's provisional diagnostic criteria. CSDD was used to evaluate depression. Only 60 patients have completed the study. There were no statistically significant differences between the two groups in terms of depression or cognitive performances. The side effects were also not significantly different when the groups were compared and there were no serious side effects reported. Therefore, it is considered that escitalopram was well tolerated by patients with AD and associated depression [28].
Studies with multiple antidepressants
According to the inclusion criteria, 6 studies have been selected to target the efficacy and tolerability of two or more antidepressants when treating AD associated depression. In four studies the antidepressants were compared one to another without a placebo group. Two studies included a control group and compared the antidepressants with one another as well as individually with the placebo group (Table 2).
| Sl no. | First author | Year | Antidepressant | Study design | Duration (weeks) | Sample size |
|---|---|---|---|---|---|---|
| 1 | Taragano FE | 1997 | Amitriptyline, Fluoxetine | RT, Double-Blind | 6 | 37 |
| 2 | Karlsson I | 2000 | Mianserin, Citalopram | RT, Double Blind | 12 | 345 |
| 3 | Banerjee S | 2011 | Mirtazapine, Sertraline | RCT, Double-Blind | 13 | 326 |
| 4 | Mokhber N | 2014 | Desipramine, Venlafaxine, Sertraline | RT, Double-Blind | 12 | 59 |
| 5 | Zuidersma M | 2019 | Sertraline, Mirtazapine | RCT Double-Blind | 13 | 326 |
| 6 | Cumbo E | 2019 | Vortioxetine, Escitalopram, Paroxetine, Bupropione, Venlafaxine, Sertraline | RT Double-Blind | 12 month | 108 |
Table 2: Studies with multiple antidepressants.
A study conducted in 1997 aimed to compare the efficacy and tolerability of amitriptyline and fluoxetine in AD associated depression. HAM-D scores, as an indicator of depressive symptoms severity, were significantly decreased, without identifying notable differences between the two treatment options. MMSE scores, used as an indicator of the effects of the treatments on cognition, have significantly increased, without notable differences between the two antidepressants. Amitriptyline tolerability was significantly lower than that of fluoxetine as indicate by the higher drop-out rate [29].
The study of Karlsson et al., assessed the efficacy and tolerability of citalopram and mianserin in the treatment of depression associated with AD. Depressive symptoms were assessed with MADRS, whereas cognitive status with MMSE. The study included 345 elderly patients with depression and mild to moderate forms of AD, as well as patients without dementia.
Patients with dementia had a lower response rate to antidepressant treatment compared to the patients without dementia, however without notable differences between the two treatment options. 13% (N=21) of the patients treated with citalopram discontinued the study, and 17% (N=30) of the patients treated with mianserin. The incidence of side-effects was relatively low in both treatment groups taking into account the advanced age of the patients. To conclude, both citalopram and mianserin were well tolerated. However, due to the lack of a placebo group, the study can present only the differences between the two antidepressants [30].
The main objective of Banerjee et al. study was to evaluate the efficacy of sertraline and mirtazapine in the treatment of depression associated with AD over a period of 13 weeks. Depression was assessed through CSDD scoring. The secondary endpoints of the study have been: the efficacy after 39 weeks of treatment, the drop-out rate and side-effects ratio as a measure of tolerability. The best treatments response was registered in the placebo group, followed by the mirtazapine group and lastly, the sertraline group. The 39-week follow-up no longer identified the significant differences between the three groups. The drop-out rate and the frequency of side-effects was relatively high but comparable between both treatment groups as opposed to the control group. Therefore, the treatment with neither sertraline, nor mirtazapine brings any benefits for the depression associated with AD. In both cases, the tolerability compared to placebo was lower [31].
A fourth study aimed to assess the efficacy of three antidepressants for patients with AD and depression. The latter was assessed with the help of HAM-D, whereas the effect of the treatment on cognition was evaluated with MMSE. HAM-D scores registered at 4, 8 and 12 weeks showed a significant improvement in favour of the sertraline group, followed by the venlafaxine group and lastly the desipramine group. Sertraline had the least effect on the cognitive status compared to the groups treated with desipramine, respectively venlafaxine [32].
In 2019 Zuidesma et al., reviewed the results of Banejee et al., study published in 2011. Having the same sample of patients as well as methodology, the conclusions of the study were similar [33].
Another 2019 study evaluated the effectiveness and tolerability of vortioxetine in comparison to several other antidepressants: escitalopram, paroxetine, bupropion, venlafaxine, and sertraline on 108 patients with AD and depression. The study did not include a placebo group. MMSE, Attentive Matrices, Raven and Coloured Progressive Matrices, as well as Digit Span were used in order to evaluate vortioxetine’s effects on cognition, the main objective of the study. HAM-D and CSDD were applied to evaluate vortioxetine ’ s effect on associated depression. Both cognition as well as the associated depression of the patients with AD were positively influenced among the vortioxetine group. Good tolerability was also present [34].
Efficacy and safety of antidepressants classes included
The results of the selected studies are at times inconclusive and even conflicting. Despite this fact, by analyzing these results, we are trying to evaluate the efficacy and safety of the antidepressant classes included and also to identify whether one of these antidepressants is more likely to have acceptable efficacy and safety for depression in AD.
Tricyclic and tetracyclic antidepressants
Imipramine, clomipramine and maprotiline were individually evaluated in studies with one antidepressant only, whereas amitriptyline, desipramine and mianserine were evaluated in comparative studies without a placebo group. Their efficacy on depressive symptoms associated with dementia was proved in almost all studies, with the exception of Reifler et al., study. The cognitive status was not positively influenced over the course of treatment, as expected, given the anticholinergic effects of these antidepressants. Tricyclic and tetracyclic antidepressants had a lower tolerability than selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs). [15,18,17,29,30,32].
Selective serotonin reuptake inhibitors (SSRIs)
Sertraline was one of the most targeted antidepressants pertaining to this class, with five single antidepressant studies and four comparative ones, out of which two of them used the comparison with a placebo group. In four out of nine studies, sertraline was shown to be effective in improving depression symptoms, while in another five studies the effect was identical to placebo. None of these studies showed any negative effects on the cognitive status and no decrease in tolerability [20,23-26,31-34].
Citalopram and Escitalopram were included in five studies, in three of them being compared to placebo, and in other two being compared to other antidepressants. One study reported its efficacy in treating AD associated depression, whereas the remaining four studies did not prove the utility of these antidepressants. In four out of the five studies tolerability was showed to be good, however in the remaining one it was not evaluated [16,27,28,30,34].
Fluoxetine had been included as a single antidepressant study as well as a comparative multiple antidepressant study, one for each of them. The single antidepressant study has proven a significant improvement of the depressive symptoms; however, the effect did not seem to last over time. The study comparing to amitriptyline had fluoxetine efficacy proven but it did not include a placebo group. Both studies describe no further cognitive alternation and good tolerability [5,16,30].
Paroxetine has been included in one comparative study among multiple antidepressants (Vortioxetine, Escitalopram, Bupropion, Venlafaxine, Sertraline) without a placebo group. The effectiveness of paroxetine was not found to be significant, however it has good tolerability [34].
Serotonin-norepinephrine reuptake inhibitors (SNRIs)
There are three studies which have targeted the use of venlafaxine for treating depression associated with AD. One of these studies compared venlafaxine with placebo, however on a sample size of 31 patients. The remaining ones have compared venlafaxine with desipramine and sertraline, respectively with vortioxetine, escitalopram, paroxetine, bupropion and sertraline. Neither of the last 2 studies did not have a placebo group. The efficacy of venlafaxine was not confirmed, and if it were present it would still be inferior to sertraline. Tolerability was also shown not to be favourable either [22,32,34].
Noradrenergic and specific serotonergic antidepressants (NaSSAs)
Mirtazapine was identified in one study where it was compared to sertraline on a significant sample size of 326 patients. Both antidepressants were proved to be effective in reducing depressive symptoms compared to placebo, but tolerability was modest [31].
Reversible inhibitors of monoamine oxidase A (RIMAs)
Moclobemide was proved to be effective in treating AD associated depression, being well tolerated and with favourable outcomes on the cognitive status. Despite having these conclusions drawn based on a single study, given the large number of cases included (N=694), the confidence intervals can be considered significant enough. This particular study ’ s limitation consists of the inclusion of elderly patients with cognitive decline and depression, without a confirmed AD diagnosis. [19].
Serotonin modulators and stimulators (SMS)
Vortioxetine was included in single comparative study with five antidepressants. The study showed a favourable effect of vortioxetine over AD associated depression. With the lack of a placebo group, however, these results can be questioned. Good tolerability was proven including the effect on cognitive performances [34].
Discussion
Most of the selected studies had a relatively small sample size. Even more so, patients have also been divided, according to each study design, into two or more groups. Having less restrictive selection criteria, the overall number of patients was not taken into consideration as an inclusion criterion.
The cases that have been included in the selected studies as well as the assessment tools used are to an extent heterogeneous, making difficult the possibility of a quantitative analysis of the results and therefore the qualitative analysis occurred as being more feasible. The lack of the patients’ homogeneity is partially caused by their diagnosis: “Alzheimer's disease”, “Alzheimer’s dementia”, “probable Alzheimer’s disease”, “mixed dementia”, “ dementia ” , “ cognitive decline in the elderly ” . Taking into account that the most frequent form of dementia is Alzheimer’s disease the patients ’ homogeneity becomes more acceptable. According to the Consortium to Establish a Registry for Alzheimer's Disease (CERAD), the prevalence of the main forms of dementia are as follows: Alzheimer’s disease – 55%, Vascular dementia – 20%, Lewy Body dementia – 15%, Fronto-temporal dementia – 5% [35].
Another aspect to be mentioned is that despite the majority of the patients having a “Major Depressive Episode” diagnosed, in other studies the depressive symptoms were diagnosed as “minor depression”, even more so, as “emotional disturbance”.
Lastly, when evaluating quantitatively the results, the use of different assessment tools for objectifying the depressive symptoms is necessary. Among the scales that been used, HAMD was found in the majority of the studies but in different forms, with 17, respectively 21 items. CDSS as well as MADRS were applied. When evaluating the cognitive status, MMSE was used in most studies, even if other clinical scales were also applied.
Our findings are being supported by several reviews and metaanalysis that have been published in recent years. A 2015 review concludes that antidepressants that have been studied so far do not show to have a clear benefit when treating AD associated depression [9]. A meta-analysis published in 2017 agrees on the lack of clear evidence of the efficacy of antidepressants for managing depressive symptoms associated to AD [13]. A second meta-analysis published in 2018 emphases that despite depression being frequent among AD patients, and that the majority of them have antidepressants prescriptions, their effectiveness is little backup by literature. This conclusion was drawn based on the little to no improvement of the assessment tools scores when evaluating depression. Despite the fact that the remission rates advocate for the utility of antidepressants in this particular pathology, the differences between different therapeutic agents and placebo have been modest. There is also some evidence supporting the increased rates of adverse effects when adding antidepressants to the pharmacological therapy [14]. Finally, a meta-analysis published in 2019 shows that the RCT’s synthetized in both systematic reviews and meta-analysis over the past 10 years lead to the conclusion that the available data does not support up until now the benefits of antidepressants for patients with associated depression that present neurocognitive disorders [36].
Conclusion
In order to obtain solid evidence of the benefit of the use of antidepressants in the treatment of AD associated depression there is a need of a higher number of randomized, double-blind, placebo-controlled clinical trials, with a rather rigorous and similar methodology, an increased sample size as well as homogeneity between patients. As for the present moment, the small number of studies and various differences among them regarding the assessment tools of the efficacy and safety of the therapeutic agents that have been studied, make it highly challenging to provide clear evidence for the antidepressant treatment for the AD related depression.
This study cannot confirm the efficacy of antidepressants for this category of patients. However, SSRIs, specifically sertraline, having an acceptable tolerability profile in spite of its unclear efficacy, would be the primary therapeutic agent indicated for depression in AD. Tri and tetra-cyclic antidepressants, having a controversial efficacy and reduced tolerability, would be advised to be avoided.
Key-points
i) Review that focuses on the efficacy and safety of antidepressant treatment in Alzheimer ’ s disease related dementia.
ii) The data available on the safety of antidepressant treatment in dementia-associated depression doesn ’ t justify their high prescription rate.
iii) The efficacy and safety profiles of antidepressant suggest that SSRI’s are indicated in Alzheimer’s disease related depression.
Declarations
No sources of funding were used to conduct this study or prepare this manuscript.
Conflict of interest
There are no conflicts of interest relevant to the content of this article.
Ethical approval
Not applicable for this review.
REFERENCES
- Geda YE, Schneider LS, Gitlin LN, Miller DS, Smith GS, Bell J, et al. Neuropsychiatric symptoms in Alzheimer's disease: past progress and anticipation of the future. Alzheimers Dement. 2013;9(5): 602-608.
- Lee HB, Lyketsos CG. Depression in Alzheimer’s disease: heterogeneity and related issues. Biol Psychiatry. 2003;54(3): 353-362.
- Teri L, Logsdon RG, Uomoto J, McCurry SM. Behavioral treatment of depression in dementia patients: a controlled clinical trial. J Gerontol B Psychol Sci Soc Sci. 1997;52(4): P159-P66.
- Rao V, Lyketsos CG. The benefits and risks of ECT for patients with primary dementia who also suffer from depression. Int J Geriatr Psychiatry. 2000;15(8): 729-735.
- Keller MB. Citalopram therapy for depression: a review of 10 years of European experience and data from US clinical trials. J Clin Psychiatry. 2000;61(12): 896-908.
- Bains J, Birks J, Dening T. Antidepressants for treating depression in dementia. Cochrane Database of Systematic Reviews. Cochrane Database Syst Rev. 2002;21(4): CD003944.
- Modrego PJ. Depression in Alzheimer's disease. Pathophysiology, diagnosis, and treatment. J Alzheimers Dis. 2010;21(4): 1077-1087.
- Leong C. Antidepressants for depression in patients with dementia: a review of the literature. Consult Pharm. 2014;29(4): 254-263.
- Siarkos KT, Katirtzoglou EA, Politis AM. A review of pharmacological treatments for depression in Alzheimer’s disease. J Alzheimers Dis. 2017;58(3): 725-733.
- Thompson S, Herrmann N, Rapoport MJ, Lanctôt KL. Efficacy and safety of antidepressants for treatment of depression in Alzheimer's disease: a metaanalysis. Can J Psychiatry. 2007;5(4): 248-255.
- Nelson JC, Devanand DP. A Systematic Review and Meta-Analysis of Placebo-Controlled Antidepressant Studies in People with Depression and Dementia. J AmeGeriatr Soc. 2011;59(4): 577-585.
- Kok RM, Nolen WA, Heeren TJ. Efficacy of treatment in older depressed patients: a systematic review and meta-analysis of double-blind randomized controlled trials with antidepressants. J Affect Disord. 2012;141(2): 103-115.
- Orgeta V, Tabet N, Nilforooshan R, Howard R. Efficacy of antidepressants for depression in Alzheimer’s disease: systematic review and meta-analysis. J Alzheimers Dis. 2017;58(3): 725-733.
- Dudas R, Malouf R, McCleery J, Dening T. Antidepressants for treating depression in dementia. Cochrane Database of Sys Rev. 2018;8(8): CD003944.
- Reifler BV, Teri L, Raskind M, Veith R, Barnes R, White E, et al. Double-blind trial of imipramine in Alzheimer’s disease patients with and without depression. Am J Psychiatry. 1989;146(1): 45-49.
- Nyth AL, Gottfries CG. The clinical efficacy of citalopram in treatment of emotional disturbances in dementia disorders A Nordic multicentre study. Br J Psychiatry. 1990;157: 894-901.
- Fuchs A, Hehnke U, Erhart C, Schell C, Pramshohler B, Danninger B, et al. Video rating analysis of effect of maprotiline in patients with dementia and depression. Pharmacopsychiatry. 1993;26(2): 37-41.
- Petracca G, Tesón A, Chemerinski E, Leiguarda R, Starkstein SE. A double-blind placebo-controlled study of clomipramine in depressed patients with Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 1996;8(3): 270-275.
- Roth M, Mountjoy C, Amrein R, Group ICS. Moclobemide in elderly patients with cognitive decline and depression: an international double-blind, placebo-controlled trial. Br J Psychiatry. 1996;168(2): 149-157.
- Magai C, Kennedy G, Cohen CI, Gomberg D. A controlled clinical trial of sertraline in the treatment of depression in nursing home patients with late-stage Alzheimer's disease. Am J Geriatr Psychiatry. 2000;8(1): 66-74.
- Petracca GM, Chemerinski E, Starkstein SE. A double-blind, placebo-controlled study of fluoxetine in depressed patients with Alzheimer's disease. Int Psychogeriatr. 2001;13(2): 233-240.
- Cunha VUG, Rocha FL, de Melo RÁ, Valle EA, de Souza Neto JJ, Brega RM, et al. A placebo-controlled double-blind randomized study of venlafaxine in the treatment of depression in dementia. Dement Geriatr Cogn Disord. 2007;24(1): 36-41.
- Lyketsos CG, DelCampo L, Steinberg M, Miles Q, Steele CD, Munro C, et al. Treating depression in Alzheimer disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction: the DIADS. Arch Gen Psychiatry. 2003;60(7): 737-746.
- Weintraub D, Rosenberg PB, Martin BK, Frangakis C, Mintzer JE, et al. Sertraline for the treatment of depression in Alzheimer disease: week-24 outcomes. Am J Geriatr Psychiatry. 2010 Apr;18(4): 332-340.
- Rosenberg PB, Drye LT, Martin BK, Frangakis C, Mintzer JE, Weintraub D, et al. Sertraline for the treatment of depression in Alzheimer disease. Am J Geriatr Psychiatry. 2010 Feb;18(2): 136-45.
- Drye LT, Martin BK, Frangakis CE, Meinert CL, Mintzer JE, Munro CA, et al. Do treatment effects vary among differing baseline depression criteria in depression in Alzheimer's disease study±2 (DIADS-2)? Int J Geriatr Psychiatry. 2011;26(6): 573-583.
- Choe YM, Kim KW, Jhoo JH, Ryu SH, Seo EH, Sohn BK, et al. Multicenter, randomized, placebo-controlled, double-blind clinical trial of escitalopram on the progression-delaying effects in Alzheimer's disease. Int J Geriatr Psychiatry. 2016;31(7): 731-739.
- An H, Choi B, Park KW, Kim DH, Yang DW, Hong CH, et al. The effect of escitalopram on mood and cognition in depressive Alzheimer’s disease subjects. J Alzheimers Dis. 2017;55(2): 727-735.
- Taragano FE, Lyketsos CG, Mangone CA, Allegri RF, Comesaña-Diaz E. A double-blind, randomized, fixed-dose trial of fluoxetine vs. amitriptyline in the treatment of major depression complicating Alzheimer's disease. Psychosomatics. 1997;38(3): 246-252.
- Karlsson I, Godderis J, Augusto De Mendonça Lima C, Nygaard H, Simányi M, et al. A randomised, double-blind comparison of the efficacy and safety of citalopram compared to mianserin in elderly, depressed patients with or without mild to moderate dementia. Inter J Geriatr Psychiatry. 2000;15(4): 295-305.
- Banerjee S, Hellier J, Dewey M, Romeo R, Ballard C, Baldwin R, et al. Sertraline or mirtazapine for depression in dementia (HTA-SADD): a randomised, multicentre, double-blind, placebo-controlled trial. Lancet. 2011;30;378(9789): 403-411.
- Mokhber N, Abdollahian E, Soltanifar A, Samadi R, Saghebi A, Haghighi MB, et al. Comparison of sertraline, venlafaxine and desipramine effects on depression, cognition and the daily living activities in Alzheimer patients. Pharmacopsychiatry. 2014;47(4): 131-140.
- Zuidersma M, Chua K-C, Hellier J, Voshaar RO, Banerjee S. Sertraline and mirtazapine versus placebo in subgroups of depression in dementia: findings from the HTA-SADD randomized controlled trial. Am J Geriatr Psychiatry. 2019 Sep;27(9): 920-931.
- Cumbo E, Cumbo S, Torregrossa S, Migliore D. Treatment effects of vortioxetine on cognitive functions in mild Alzheimer’S disease patients with depressive symptoms: a 12 month, open-label, observational study. J PrevAlzheimers Dis. 2019;6(3): 192-197.
- Gearing M, Mirra S, Hedreen J, Sumi S, Hansen L, Heyman A. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part X. Neuropathology confirmation of the clinical diagnosis of Alzheimer's disease. Neurology. 1995;45(3): 461-466.
- Bingham KS, Flint AJ, Mulsant BH. Management of late-life depression in the context of cognitive impairment: a review of the recent literature. Curr Psychiatry Rep. 2019;21(8): 74.
Citation: Buciuta A, Vinasi RC, Coman HG (2020) Antidepressant Treatment for Depression in Alzheimer’s Dementia: Systematic Review Article. J Aging Sci. 8: 229. Doi: 10.35248/2329-8847.20.08.229.
Copyright: © 2020 Buciuta A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.