PMC/PubMed Indexed Articles
Indexed In
- Open J Gate
- Academic Keys
- JournalTOCs
- ResearchBible
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review - (2019) Volume 7, Issue 3
A Mini Review of Astaxhantin and Oxidative Stress in Aging
Gülsüm Deveci* and Nilüfer Acar TekReceived: 09-Sep-2019 Published: 01-Oct-2019
Abstract
There are more than 300 theories attempting to explain old age or senescence, which is affected by genetic and environmental factors. Of all these theories, with respect to free radical theory, oxygen species occurring in the wake of extrinsic and intrinsic factors may trigger senescence and accelerate the aging process. Another reason for free radical generation is decrement of antioxidants and its components intake in diet. Astaxhantin (ASTX) has more antioxidant activity than other carotenoids. Because of this property, astaxhantin may be a substantial antioxidant source in the diet. In this review, we discuss antioxidant effects of astaxhantin on potential mechanisms in senescence.
Keywords
Astaxhantin; Oxidative stress; Senescence
Introduction
Free radicals, wear and tear, destructive DNA damage, mechanism loss of mitochondria, cellular adaptation and stochastic theories are some of over 300 theories about the aging process [1-4]. With regard to these theories, exogenous factors, such as lifestyle, stress, contribute to aging process, in addition to some metals and naturally occurring free radicals in cells and tissues [1,5].
Lots of agents such as hormones, body proteins and immune system cells also change with age as a result of diversity in gene expression [1]. Diminished physiological function leads defense system up against oxidation and other biochemical variance to fall into a decline and cell survival to become shorter [6].The other reason for oxidative stress increasing with age is decreased intake of exogenous antioxidants from nutrition [7].
Utilization of nutritional supplements against inadequacies due to nutritional diversification seen in the elderly lessens the adverse effects of senescence on immune functions [8]. Antioxidants such as ascorbic acid, phenolic compounds, sterols and carotenoids, which are indigenous to foods, decrease oxidative damage to a minimum level or/and prevent it altogether [9,10].
Various carotenoids, pigments derived from organic hydrocarbons, not only induce antioxidant activity but also have positive effects on glutathione and activation of enzymes such as cyclooxygenase, SOD and caspase-3 and -9 [11-15].There are two subunits of carotenoids in terms of oxygen atom-inclusiveness, carotenes, and xanthophyll’s [11]. Astaxhantin (ASTX) is an oxidized carotenoid derivative, including both hydroxy and oxy groups [16].
Additionally, double band and polar-apolar-polar structure help differentiate ASTX from other xanthophyll’s and contribute to greater antioxidant activation [17]. Other properties that make ASTX special include indicating vitamin A activity in due course of metabolized in liver, permeant through blood-brain barrier and partaking in grey matter of brain [18,19].
Abdelzaher et al. suggested that ASTX inhibits reactive oxygen species [20]. They stated that incubation of 25 μM ASTX in cellculture for 24 hours reduces accumulation of free radicals in cells [21]. In another study, individuals aged 20-55 years who consumed 20 mg ASTX for 12 weeks experienced decreased serum malondialdehyde levels, an indicator of oxidation [22]. Astaxhantin administrations in various doses in rats and humans (20 mg/kg/day in rats; 5 mg/day, 20 mg/day, 40 mg/day in humans) provide superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) with activation and enhance total antioxidant capacity [23-25].
Baralic et al. seeking to understand the effect of ASTX on oxidative stress in humans has shown that subjects given 4 mg ASTX for 90 days had increased paraoxonase-1 activity, an antioxidant enzyme [26]. In this article, because of the unique properties of ASTX that distinguish it from other carotenoids, and because it is high in antioxidants, we investigated impacts of ASTX on oxidative stress dependent on senescence, and is tackled that it may be protective against oxidative stress.
Materials and Methods
Properties of Astaxhantin
Hydroxy (OH-) groups in both two terminal ends of ASTX make polar characteristic available to its pattern while middle part is also nonpolar [27]. Chemical structures of ASTX and its metabolites were shown in Figure 1 [28].
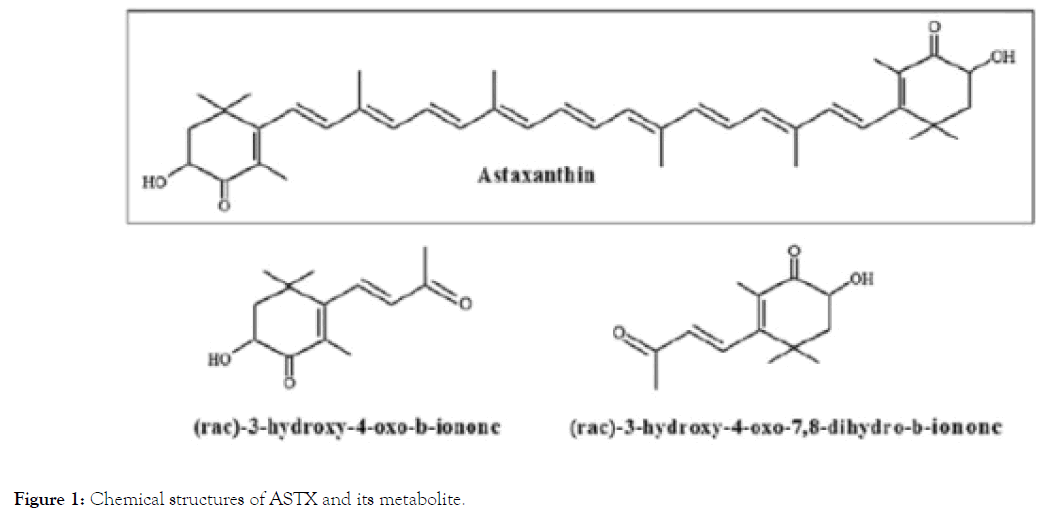
Figure 1. Chemical structures of ASTX and its metabolite.
There are several isomers subject to configuration of OH- groups [17]. Natural ASTX is esterified and artificial ASTX is free form [29]. Astaxhantin stereoisomers are classified by their OHgroups. Astaxhantin isomers are Enantiomer (3S, 3’S), Enantiomer (3R, 3’R), and Mesomere (3R, 3’S) that are identified for C-3 and C-3’ carbon atoms in chiral-cores. Of these isomers, enantiomer (3S, 3’S) is the dominant form of ASTX (Figure 2) [30,31].
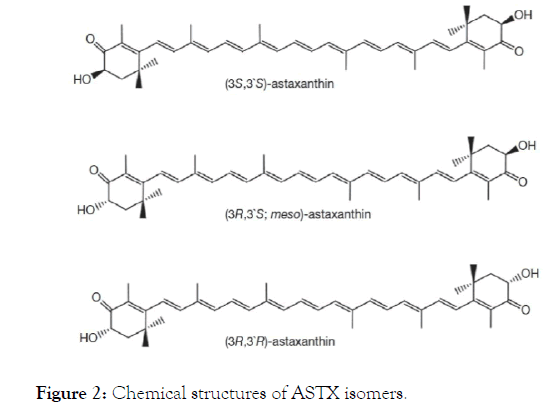
Figure 2. Chemical structures of ASTX isomers.
Once consumed, astaxhantin is first absorbedviapassive diffusion by enterocytes, and is got undergone via facilitated diffusion in the presence of lipids [29]. Its non-esterified form is brought in through chylomicrons to the liver. Then, this form is transported by lipoprotein to other tissues [32]. More than 50% of ASTX taken within 24 hours is metabolized. The main metabolic afterproduct is 3-hydroxy-4-oxo-β-ionone. Afterwards, this last product conjugates to 3-hydroxy-4-oxo-7,8-dihydro-β- ionone (Figure 1) [28,33]. Basic sources of ASTX are phytoplankton and Haematococcus pluvialis H. (pluvalis) in addition to yeast, some mushrooms, bacteria, shellfish, krill, shrimp, salmon, lobster, and some plants [18].
The Food and Drug Administration (FDA) has predicated ASTX grade got H.pluvalis in Generally Recognized As Safe (GRAS) since 2010. It is suggested that consumption of up to 40 mg/d in humans doesn’t have adverse effects according to this list. Natural ASTX Complex, including extract of H.pluvalis, common fatty acids, and other carotenoid esters is recommended at a dose of 2–12 mg/day (mean dose 6 mg/d) by the FDA [34]. There are many products in the market as ASTX supplements (Table 1).
| Products | Ingredients | Origin of ASTX | Amounts of ASTX | Advised daily intake | Reference |
|---|---|---|---|---|---|
| ZanthoSyn® | Modified food starch, vegetable celulose capsule, microcrystalline, cellulose, vegetable stearate, corn starch, glucose syrup, sodium ascorbate and DI-alpha-tocopherol. | - | 12 mg | 1 capsule | https://www.gnc.com/gnc-exclusive-brands/539600.html |
| Solgar® | Sunflower seed oil, gelatin, vegetable glycerin, lutein, canthaxanthin and beta carotene. | H.pluvialis | 10 mg | 1 softgel | https://www.gnc.com/other-antioxidants/216780.html?cgid=other-antioxidants |
| 5 mg | 1 softgel | https://www.gnc.com/other-antioxidants/216781.html?cgid=other-antioxidants | |||
| Lıfe Extensıon® | Phospholipids, sunflower oil, gelatin, glycerin, purified water and extra virgin olive oil. | H.pluvialis | 4 mg | 1-2 softgel | https://www.gnc.com/other-antioxidants/215995.html?cgid=other-antioxidants |
| AstaPure® | Cold-pressed extra-virgin oil and capsule shell (gelatine, glycerol, beta-carotene, caramel E150a). | H.pluvialis | 4 mg | 1-2 capsule | https://www.amazon.co.uk/Essentials-Astaxanthin-Astapure-Delivering-Capsules/dp/B07BGHKG77 |
| Extra virgin olive oil and vegetarian softgel (modified food starch, glycerin, carrageenan, purified water). | H.pluvialis | 6 mg | 1 softgel | https://www.amazon.co.uk/Doctors-Best-Astaxanthin-AstaPure-Softgels/dp/B0 0KMX35P2 | |
| AstaReal® | Extra virgin olive oil, gelatin (bovine), glycerin and purified water. | H.pluvialis | 6 mg | 1 softgel | https://www.drvita.com/products/natures-lab-astareal-astaxanthin-6mg-60-softgels |
| BioastinTM | Safflower oil, gelatin, glycerin, purified water and natural tocopherols. | H.pluvialis | 12 mg | 1-3 gel caps | https://www.pureformulas.com/bioastin-natural-astaxanthin-60-capsules-by-nutrex-hawaii.html |
Table 1: ASTX products being in market.
Oxidative stress in senescence
Sections of DNA related to aging, progressive faults of protein synthesis, immune attacks in developed organisms against selfantigens, and free radical damage are assumed in the nature of the aging process [35]. Antagonistic pleiotropy theory, fallout accumulation theory, insulin resistance, advanced glycation, wrong destruction theory, autoimmunity, circadian rhythm, and evolutionary theory are some of the propounded theories. Of these theories, free radical theory is involved in both genetic mutations and aggregation of cellular catabolites [36]. Although the aging process and its mechanisms have not been clarified yet, of attractive aging theories, wear and tear, genetic programming telomere, pace of life, mitochondria, endocrine and wrong destraction theory, hypothesis of advanced DNA damage and free radical theory are updated [37].
Harman (1956) has suggested that accrescent free radicals in the wake of molecular oxygen catalyzed in cells cause senescence by their side effects on cells and tissues [5]. Free radicals, forming from exogenic and endogenic resources in cells, have detrimental effects on both the cell nucleus and genomes of mitochondrial DNA [38]. It is asserted that environmental factors, genetic modification, diseases, and naturally-occurring free radicals in aging trigger senescence [39].
There are produced reactive oxidative species in the course of diverse cell reactions. These species are undergone detoxification by enzymes and antioxidant compounds (Figure 3) [40].
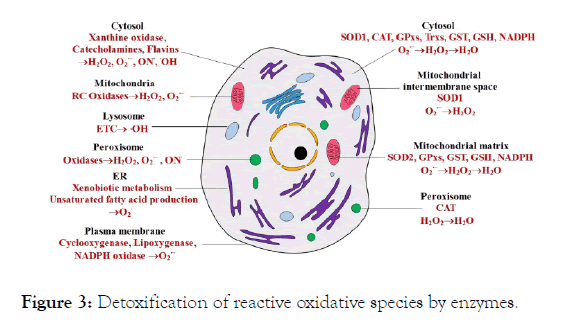
Figure 3. Detoxification of reactive oxidative species by enzymes.
It is discussed that damage signaling pathways increase in company with aging situation, decreasing general physiological capacity and increasing mortality. One of these pathways is DNA damage response (DDR). Inadequency and defectiveness in DDR as well as accumulation of DNA lesion trigger aging and pathological diseases associated with aging. p53 tumor suppressor protein expressed in responsed to DNA damage modulates cascade I and therefore it is accepted as an important DD [41].
Activity of Nrf2 being another DDR play a repressive role in numerous steps of aging and diseases related with aging. Nrf2 also creates a response by increasing expression of detoxification genes [42].
The longevity-modulating genes found out past two years are preserved by from smallest living to human. Of these genes, Cell aging regulation system (CARS) includes diverse aging effectors modulating longevity, such as lipid unsaturation, mitROS production, mitochondrial DNA repair, autophagy, apoptosis, proteostasis or telomere shortening. Because of these properties, CARS is inclusory such as to previous theories [43].
Mitochondria is origo of intracellular free radical species. Mitochondrial DNA (mtDNA) is exposed to grave oxidative stress. An important oxydant, 8-hydroxydeoxyguanosine (8OHdG), provokes erroneous conjugation and point mutations. Due to the fact that disruptions in mtDNA based on these errors may ocur, it is assumed that mtDNA damage is a reason for aging [44]. Mitochondria theory is descriptive of senescence as increased production of free radicals due to disorders in the mitochondria electron transport chain and premediates that aging is significantly alleviation in enzymes, especially transporter enzymes, with age [45]. One study (2016), seeking out relationship between hippocampal proteins and age, found that proteins in electron transport chains and fusion pathways of synaptic vesicles consistently decrease with age [46]. Unpaired protein-2 (UCP-2), a mitochondrial transporter protein, may have an impact on life span in humans. In reference to this consideration, rats without UCP-2 matureity earlier and have a shorter lifetime [47]. A study (2011) that investigated impacts of senescence on activity of the mitochondria electron transport chain showed that the most reduction is seen in activity of complex-4 while complex-2 does not change [48]. Senescence is associated with oxidative stress and accumulation of mutant mtDNA. Hydrogen peroxide level, releasing of NADPH oxidase-2 (NOX-2), 8OHdG and accumulation of mutant mtDNA increase and caspase-3- dependent apoptosis pathway in a study of Du et al. working on old rats [49]. Dysfunction of Nrf2 in vessels further augments oxidative stress levels occurring with age [50,51], going into diverseness in response of fibroblasts human of old and young to oxidative stress, found that expression of phase-2 enzymes, such as CAT and SOD, is greater in younger individuals than in older [51]. They noted that lifespan of rats without SOD is substantially shorter and these rats age quickly [52].
Based on alterations in mitochondria, diseases with aging, such as Alzheimer’s disease (AD), Parkinson disease (PD), amyotrophic lateral sclerosis (ALS) and Huntington's disease (HD), come in sight. Of these diseases, accumulations of Aβ protein in AD affect Complex-IV and activity of α-ketoglutarate dehydrogenase. This protein also binds to Aβ-binding alcohol dehydrogenase. Of another disease, Lewy bodies accumulate in mitochondria and decrease Complex-I activity in PD. In another disease, ALS, it is a matter of abnormal outturn of mitochondrial ROS depending on SOD 1 mutations. In HD, there is a decrease in Complex-II. All of these mitochondrial disfunctions by diseases are shown in Figure 4 [53].
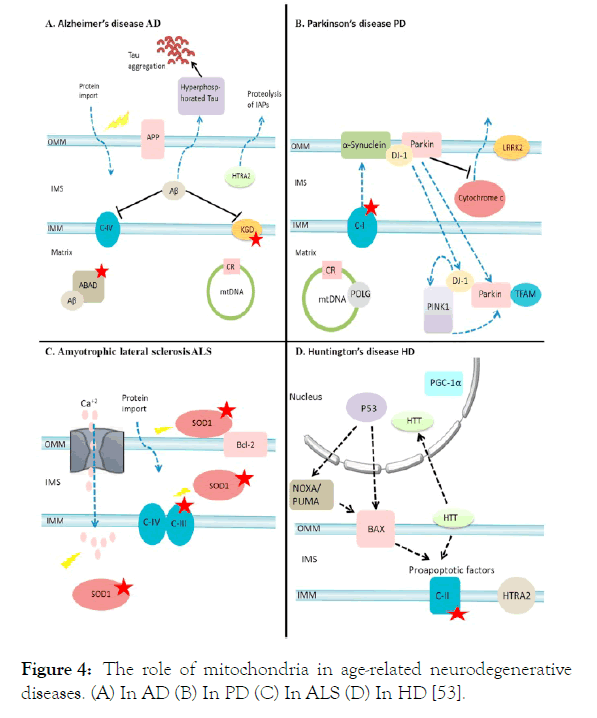
Figure 4. The role of mitochondria in age-related neurodegenerative diseases. (A) In AD (B) In PD (C) In ALS (D) In HD [53].
Effects of Astaxhantin on oxidative stress
β-carotene and vitamin C, being antioxidant, are located in both inside and outside of cell lipid layer of membrane. However, besides participation in the double-layer membrane, the presence of the cell both inside and outside gives ASTX better protection than others (Figure 5) [54].
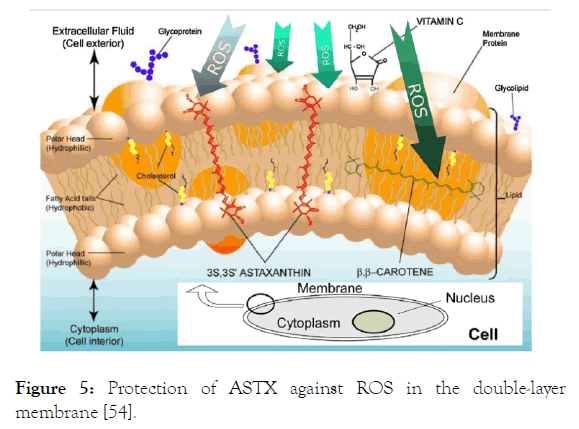
Figure 5. Protection of ASTX against ROS in the double-layer membrane [54].
It is adduced in studies related to astaxhantin that ASTX decreases oxidant stress by inducing antioxidant enzymes, such as Nrf2, PI3K/Akt, SOD and glutathione, and their pathways [55-58].
It is separately researched as mice, human and cell studies.
Results and Discussion
Mice studies
Effects ASTX on antioxidant enzymes: Al-Amin et al. were studied on mice to measure the levels of anti-oxidative enzymes in the prefrontal cortex (PFC), striatum (ST), hippocampus (HP), and liver (LV) in groups [59]. Treatment of 2 mg/kg ASTX for 28 days induced CAT (in ST and LV) and SOD (ST, HP and LV) while supressed nitric oxide (in PCF, ST, HP and LV), glutathione (in LV) [59]. To investigate effects of ASTX on antioxidant enzymes, 10 mg/kg ASTX were given for 28 days and 100 mg/kg ASTX were given for 7 days to rats and, as a result, SOD activity increased for both dose levels [60,61]. Al- Amin et al. gave 2 mg/kg/day ASTX to old rats and found that activities of CAT and SOD and glutathione levels increased at the end of the study [62].
In a study on adult Wistar male rats for 45 days that Mattei et al. carried on effect of ASTX on oxidative stress due to fish-oil, while only fish-oil (10 mg EPA/kg body weight and 7 mg DHA/kg body weight) decreased activities of SOD, CAT and glutathione reductase, ASTX administration (1 mg/kg body weight) after fish-oil improved these activities [63]. Otsuka et al. investigated effect of ASTX on photoreceptor degeneration induced by fluorescent and noted 8-OHdG level increased in outer core layer. After ASTX administration (100 mg/kg, at 6 h before and at 0, 6, 12, 24, 36, 48, and 72 h after light irradiation) to male albino ddY mice, there was no effect of ASTX on mRNA synthesis of Sod1, metallotionein-II and II [64]. ASTX administration of 100, 250 and 500 mg/kg body weight to ulcerated albino Wistar rats promoted SOD and CAT levels [65].
Al-Amin et al. administrated ASTX (20 mg/kg body weight/day) to Swiss albino male mice for 42 days. Glutathione concentrations of frontal cortex, striatum and cerebellum decreased after administration. In addition to this, malondialdehyde levels diminished in striatum, hypothalamus and hippocampus [66]. Superoxide anion levels in neutrophile of alloxane induced diabetic Wistar male rats fed ASTX (20 mg ASTA/kg of body weight/day, for 30 days) did not change, while there was a diminishment in TBARs levels. At the same time, this administration increased GPx level further [67].
Effects ASTX on reactive oxygen species: Pan et al., who studied adult rats, reported that ASTX given in doses of 5 and 10 mg/kg for a week suppresses reactive oxygen species and activates the defense system of antioxidants [68]. In another study (2016), oxidative stress levels were evaluated, and old rats were given 1.7 mg/day ASTX. They found that the level of plasma oxidative stress was lower while antioxidant levels were higher by the end of 72 weeks [69]. After administration of 25 and 50 mg/kg ASTX in rats, there were decreases in levels of 8OHdG and malondialdehyde [70].
Metabolic disorders such as oxidative stress, happens after high fatty diets and this diet causes neural damage and alterations in neurogenesis and memory. In a study researched effects of antioxidants (ASTX, vitamin C and E; 0,6 g/kg, 0,2 g/kg and 0,2 g/kg, respectively, for 180 days) on potential side-effect of high fatty diet, ASTX administration to male Wistar rats enhanced total antioxidant capacity [71]. In another study sought relevany between ASTX and 8-OHdG level, both 300 and 600 mg/kg/day administration for 3 weeks of ASTX to male SHRSP rats decreased urinary 8-OHdG level [72].
Yeh et al. gave 0.6 mg/kg and 3 mg/kg ASTX to old rats for 8 weeks. They noted that levels of 8OHdG, nitrotyrosine, acrolein and transcription of nuclear factor kappa B (NF-kB) decreased in both groups [73].
Effects ASTX on pathways of oxidative stress: Masoudi et al. asserted that the release of caspase-3 significantly decreased when adult rats were given 10 μL/0.2 mM ASTX [74]. In a rat study, administration of 20 μM/0.1 mM ASTX activated the Nrf2 – ARE pathway [75]. Masoudi et al. to examined the therapeutic potential of AST on adult rats after severe spinal cord injury. 10 μL/0,2 mM ASTX, ASTX reduced expression of Bax and Cleaved-caspase-3 proteins and increased expression of the Bcl-2 protein [74].
Fakhri et al. and Rahman et al. also found that ASTX lessened expression of TNF-α [76,77]. Amyloid plaques appear in pathogenesis of AD and have cytotoxic property. In a study, relation between ASTX and Bexratone was viewed by Fanaee- Danesh et al. [78]. Both two administrations had similar effect. By way of ASTX, Female 3xTg AD mice fed ASTX (80 mg/kg, for 6 days) had reduced Aβ oligomers [78]. All the mice studies mentioned in review are demonstrated in Table 2.
| Study type | Aim of study | Experimental procedure | Results | Reference |
|---|---|---|---|---|
| Swiss Albino Mice | to measure the levels of anti-oxidative enzymes in the prefrontal cortex (PFC), striatum (ST), hippocampus (HP), and liver (LV) in groups. | Group I: control (0,9% saline) | In comparison with group II, levels of nitric oxide (in PCF, ST, HP and LV), glutathione (in LV) were significantly lower and higher CAT (in ST and LV) and SOD (ST, HP and LV) in group III. | Al-Amin et al., 2019 |
| Group II: scopolamine (0,5 mg/kg) | ||||
| Group III: scopolamine (0,5 mg/kg) and ASTX (2 mg/kg) | ||||
| Duration of study: 28 days | ||||
| Male Wistar Rats | to investigate the potential effects of ASTX to modulate sensory-motor and histopathological dysfunctions, through modifying the NR2B and phospho-p38MAPK (p-p38MAPK) signaling elements as well as TNF-α, in a severe compression model of spinal cord injury (SCI) in rats. | It was separated the rats into 3 groups: Sham, SCI (treated with 5% dimethyl sulfoxide) and SCI (treated with 10 μL of 0,2 mM). | In comparison to SCI (treated with 5% dimethyl sulfoxide), ASTX treatment significantly decreased expressions of p-p38MAPK on 14-, -21 and -28 day and TNF-α on 14-day. | Fakhri et al., 2018 |
| Duration of study: 28 days | ||||
| Female Albino Rats | to search neuroprotective role of ASTX in rats with Alzheimer’s disease (AD). | Rats were divided into 5 groups as: Group I: Sham, group II: Aβ(1-42) infused (4 μg/4 μL), group III: 0,5 mg/kg/day and Aβ(1-42) infused (4 μg/4 μL), group IV: 1 mg/kg/day Aβ(1-42) infused (4 μg/4 μL), group V: 1 mg/kg/day | Comparing to group II, both doses of ASTX given with Aβ(1-42) significantly decreased level of TNF-α after 28 days. | Rahman et al., 2019 |
| Adult Rats | to examine whether pretreatment of astaxanthin can protect against ischemic injuries in the adult rats | 5 and 10 mg/kg ASTX for seven days | Administration of ASTX supressed ROS | Pan et al., 2017 |
| Female ICR Old Rats | to first determine effects of ASTX on the structural changes by oxidative stress in an aging mouse model | Administration of 1.7 mg/kg ASTX for 72 weeks | ASTX decreased oxidative stress and elevated antioxidant levels. | Kuraji et al., 2016 |
| Male Db/Db Mice | to investigate the effects of astaxanthin on diabetic retinopathy in db/db mice with the reduction of oxidative stress. | Treatment with 25 and 50 mg/kg ASTX | Both of tese doses decreased levels of 8OHdG and malondialdehyde. | Dong et al., 2013 |
| Vascular Cognitive Impairment Mice | to investigate the potential neuroprotective effect of the antioxidant astaxanthin (ATX) in the mice. | 10 mg/kg ASTX for 28 days | While the concentration of malondialdehyde was decreased, the levels of glutathione and SOD were increased by ASTX. | Xue et al., 2017b |
| Sprague-Dawley Rats | to investigate the potential effects of astaxanthin on sepsis and multiple organ dysfunctions. | 100 mg/kg ASTX for 7 days | SOD activity was increased by ASTX. | Zhou et al., 2015 |
| Adult Rats | to examined the therapeutic potential of AST on adult rats after severe spinal cord injury. | 10 μL/0,2 mM ASTX | ASTX reduced expression of Bax and Cleaved-caspase-3 proteins and increased expression of the Bcl-2 protein. | Masoudi et al., 2017 |
| Old Swiss Albino Mice | to investigate the age-dependent and region-specific antioxidant effects of astaxanthin in mice brain | 2 mg/kg/day ASTX | ASTX activated CAT and SOD and increased glutathione levels | Al-Amin et al., 2015 |
| Wistar Rats | to evaluate whether orally administered ASTX protects against oxidative damage | 0.6 mg/kg and 3 mg/kg ASTX for 8 weeks | Both doses lowered levels of 8OHdG and NF-kB. | Yeh et al., 2016 |
| Male Sprague-Dawley Rats | to assess the effect of ASTX on the Nrf2-ARE pathway | 20 μL/0.1 mM ASTX for 1 hour | It was activated Nrf2-ARE pathway. | Wu et al., 2014b |
| Swiss Albino Male Mice | to investigate effect of ASTX on oxidative stess incompliance with brain regions | Diets for 42 days (1)CON (control): – 200 ml distilled water was given. (2)AlCl3: Aluminum chloride was given at a dose of 50 mg/kg body weight per day (3)AST_AlCl3 – astaxanthin at a dose of 20 mg/kg body weight together with aluminum chloride at a dose 50 mg/kg body weight was given (4)AST – only astaxanthin at a dose of 20 mg/kg body weight was given |
Glutathione concentrations of frontal cortex, striatum and cerebellum decreased after administration. In addition to this, malondialdehyde levels diminished in striatum, hypothalamus and hippocampus. | Al-Amin et al., 2016 |
| Male Wistar Rats | to search ASTX and antioxidant capacity | Diets for 180 days Antioxidant compounds with amounts 0.2 g/kg of vitamin E, 0.2 g/kg of vitamin C and 0.6 g/kg of ASX HFD consists of 60.9% fat, 18.3% protein, and 20.3% carbohydrate,with a caloric density of approximately 5.24 kcal/g. A standard laboratory rodent chow diet (Lab Diet) was used for the control diet. This control diet has a caloric density of approximately 3.0 kcal/g. |
ASTX administration enhanced total antioxidant capacity. | Komaki et al., 2015 |
| Adult Wistar Male Rats | to analyse ASTX and antioxidant enzymes | For 45 days Four experimental groups of 16 animals each were formed: (i) control, fed with 400 μL of 10% Tween-80 aqueous solution (v/v); (ii) ASTA, fed with 1 mg ASTA/kg; (iii) FO (fed for 10 mg EPA/kg and 7 mg DHA/kg); (iv) ASTA/FO, fed with 1 mg ASTA/kg, 10 mg EPA/kg and 7 mg DHA/kg. Each fish oil (FO) capsule of 500 μL contains 9 kcal (38 kJ), 2.0 mg of mixed tocopherols, and 1.0 g of total fat, which 30% are from saturated fats, 20% from monounsaturated fats (mostly palmitoleic and oleic acids), and 50% of polyunsaturated fatty acids (180 mg EPA and 120 mg DHA). AstaREAL A1010 is an astaxanthin-rich natural Haematococcus pluvialis product that contains 5.2–5.8% of total carotenoids, whereas 5.0–5.6% are purely astaxanthin (3.9% as monoesters, 0.9% as diesters, and 0.1% in free form), 0.02% lutein/zeaxanthin, 0.02% adonirubin, 0.02% cantaxanthin, 0.02% β-carotene, and 0.1% others. |
While only fish-oil (10 mg EPA/kg body weight and 7 mg DHA/kg body weight) decreased activities of SOD, CAT and glutathione reductase, ASTX administration (1 mg/kg body weight) after fish-oil improved these activities. | Mattei et al., 2011 |
| Male Albino Ddy Mice | to seek effect of ASTX on enzymes | Mice were exposed to 8,000 lux of white fluorescent light for 3 h. Astaxanthin at 100 mg/kg was dissolved in olive oil just before use and was administered orally eight times (at 6 h before and at 0, 6, 12, 24, 36, 48, and 72 h after light irradiation). | After ASTX administration (100 mg/kg, at 6 h before and at 0, 6, 12, 24, 36, 48, and 72 h after light irradiation) to male albino ddY mice, there was no effect of ASTX on mRNA synthesis of Sod1, metallotionein-II and III. | Otsuka et al., 2013 |
| Male Shrsp Rats | to asses ASTX and 8-OHdG | 300 or 600 mg astaxanthin/kg every day, for 3 weeks | Both 300 and 600 mg/kg/day administration for 3 weeks of ASTX to male SHRSP rats decreased urinary 8-OHdG level. | Sasaki et al., 2011 |
| Albino Wistar Rats | to research ASTX and antioxidant enzymes such as SOD and CAT levels. | 100, 250 ve 500 mg/kg vücut ağırlığı ASTX, for 21 days | ASTX administration of 100, 250 and 500 mg/kg body weight to ulcerated albino Wistar rats promoted SOD and CAT levels. | Kamath et al., 2008 |
| Wistar Male Rats | to asses relation ASTX and oxidative stress parameters | (a)control, fedwith olive oil for 30 days; (b)ASTA, fed with 20 mgASTA/kg BW for 30 days; (c)diabetic, fed initially with olive oil alone for 23 days, then treated with alloxan to induce diabetes, and finally with olive oil for extra 7 days (to complete 30 supplementation days); (d)diabetic+ASTA, fed initially with 20 mg ASTA/kg BW alone for 23 days, then treated with alloxan to induce diabetes and continued with 20 mg ASTA/kg BW for extra 7 days (to complete 30 supplementation days) |
Superoxide anion levels in neutrophile of alloxane induced diabetic Wistar male rats fed ASTX (20 mg ASTA/kg of body weight/day, for 30 days) did not change, while there was a diminishment in TBARs levels. At the same time, this administration increased GPx level further. | Marin et al., 2011 |
| Female 3xtg AD Mice | to investigate impact of ASTX on Aβ oligomers | In study I,<1-year-old (32–49 weeks old) female 3xTg AD mice were gavaged for 6 days with vehicle (10% DMSO in corn oil [v/v]; vehicle control group, n=10), Bex (100 mg/kg in DMSO/corn oil; n=9), or Asx (80 mg/kg in DMSO/corn oil; n=8) and compared to non-Tg mice (37–49 weeks; n=5 for vehicle control group; n=6 for Bex; n=7 for Asx). In study II, aged (68–92 weeks old) female 3xTg AD mice were gavaged for 6 days with vehicle (n=8), Bex (100 mg/kg; n=6), or Asx (80 mg/kg; n=8). Body weights were assessed before treatment, at day 4, and before sacrification. |
(Results for study II) Both two administrations (ASTX and Bexratone) had similar effect. By way of ASTX, Female 3xTg AD mice fed ASTX (80mg/kg, for 6 days) had reduced Aβ oligomers. |
Fanaee-Danesh et al., 2019 |
| Male Sprague-Dawley Rats | to explore whether ATX treatment post SAH could activate the Nrf2-ARE pathway | Subarachnoid hemorrhage (SAH) group (n = 24); SAH + ATX group (n = 24); SAH + vehicle group (n = 24); and control group (n = 24). In the SAH + ATX group, ATX (20 μL of 0.1 mM dissolved in vehicle) was administrated at 30 min after SAH was induced. | Incubation of ASTX activated the Nrf2 – ARE pathway. | Wu et al., 2014b |
Table 2: Mice studies related with ASTX, their procedures and results.
Cell studies
Effects ASTX on oxidative stress: Yamagishi and Aihara administrated different doses of ASTX (1 nM, 10 nM and 100 nM) to rat retinal ganglion cells and found that the lifespan of cells increased, and the administration of 100 nM ASTX markedly prevented DNA damage and apoptosis [79]. In other study, it was aimed at determination of the effect of ASTX on the interplay of NRF2 and NFκB for its antiinflammatory and antioxidant properties in macrophages. In this study, treatment of macrophages cells with 25 μM ASTX for 24 hours reduced mRNA levels of IL-6, IL-1β and TNF-α [80]. In a culture study, a dose of 1.25-5 μM ASTX increased secretion of HO-1 and Nrf2 and decreased the release of caspase-3, -8 and -9 and lactate dehydrogenase. Antioxidant response components (ARE) also increased in this culture study [81].
Effects ASTX on reactive oxygen species: Another cell culture study that distinct ASTX doses (5 μM, 10 μM and 20 μM) for one hour found that production of intracellular reactive oxygen and cell death due to hydrogen peroxide were diminished [82]. Guerra et al. sought connection between glycolaldehyde and human neutrophils. Combine application of vitamin C (100 mM) and ASTX (2 mM) decreased output of NO and H2O2 in cells, unfavourable impacts of glycolaldehyde [83]. Whereas, mitochondrion contributes to energy production by redox, it also causes ROS to occur. Resultant ROS and ASTX were inspected by Wolf et al. [84]. PC12 cells incubated with 200 and 400 nM ASTX for 24 h were protected against cell death due to oxidative stress. However, 800 nM ASTX had a constructive effect on Jurkat cells. HeLa cell cultured with same dose for 6 h also provided a decrease in H2O2 levels [84]. In company with ASTX (25, 50, 100, 500 and 1000 nM, for 4 h and 24 h) cultured in human dopaminergic neuroblastoma SH-SY5Y cells, it was provided a decrease in DHA-OOH levels. ROS production was protected by ASTX in these cells [85]. CDX-085 is a pro-drug, more soluable in water than pure ASTX form. Proinflammatory ONOO- formation was reduced in platelets from C57BL/6 rats fed 0,4% CDX-085 for 2 weeks that were treated with 1 μM ASTX [86].
Fatty acids subject to concentration and type alter leucocyte function. Campoio et al. searched effects of ASTX on excess of oxidative stress based on fatty acids in human peripheral blood lymphocytes. In contrast to adverse effects of fatty acids, ASTX implementation (2 μM of ASTA for 24 h) reduced cellular NO and H2O2 production. Beside this, increasing activities in SOD, CAT and GPx by fat was lowered by ASTX [87].
Effects ASTX on enzymes: In a study on cell culture done by Franceschelli et al., horary incubation of 10 μM ASTX also induced antioxidant enzyme activation (CAT and SOD) [88]. ASTX being one of the major xanthophylls indicates an inhibitor impress on CYP isoenzymes. Astaxanthin (from 0,05; 0,5 to 5 μM) implemented on human liver microsome had an weak effect on CYP2C19 and IC50 value was 16,2 μM [52,89].
All the cell studies mentioned in review are demonstrated in Table 3.
| Study type | Aim of study | Experimental procedure | Results | Reference |
|---|---|---|---|---|
| Rat retinal ganglion cells | To investigate whether ASTX confers a neuroprotective effect against glutamate stress, oxidative stress, and hypoxiainduced apoptotic or necrotic cell death in primary cultures of rat retinal ganglion cells | Treatment with 1 nM, 10 nM, and 100 nM ASTX | DNA damage and apoptosis decreased in 100 nM ASTX treatment. | Yamagishi and Aihara, 2014 |
| HT22 cells | To investigate effects of ASTX on HT22 cells | 1,25-5 μM ASTX | Astx increased antioxidant response components, levels of HO-1 and Nrf2 while it decreased caspase-3, -8 and -9 and lactate dehydrogenase. | Wen et al., 2015 |
| U937 cells | To investigate the potential role protective of ASTX | 10 μM ASTX for 1 hour | It was induced activations of CAT and SOD. | Franceschelli et al., 2014 |
| ARPE-19 cells | To investigate the protective effect of AST on ARPE-19 cells against oxidative stress | 5, 10 and 20 μM ASTX for one hour | It was reduced intracellular ROS and cell death. | Li et al., 2013 |
| Murine RAW 264.7 macrophages | To determine determination of the effect of ASTX on the interplay of NRF2 and nfκb for its antiinflammatory and antioxidant properties in macrophages | 25 μM ASTX for 24 hours | Treatment of macrophages cells with ASTX reduced mRNA levels of IL-6, IL-1β and TNF-α. | Farruggia et al., 2018 |
| Human peripheral blood lymphocytes | To investigate effects of ASTX on excess of oxidative stress based on fatty acids in human peripheral blood lymphocytes | The cells were treated with 0.3 mM of the fatty acid mixture (the proportion of fatty acids was as follows: 1.74% lauric (C12:0), 5.2% myristic (C14:0), 31% palmitic (C16:0), 1.1% palmitoleic (C16:1), 41% stearic (C18:0), 4.6% oleic (C18:1), 9.6% linoleic (C18:2), 1.3% linolenic (C18:3), 3.2% arachidonic (C20:4), 0.45% eicosapentaenoic (C20:5), and 1.8% docosahexaenoic (C20:6) acids) added or not of 2 μM of ASTA solubilized in DMSO and cultured at 5% CO2 for up to 24 h at 37°C. | ASTX implementation reduced cellular NO and H2O2 production. Beside this, increasing activities in SOD, CAT and GPx by fat was lowered by ASTX. | Campoio et al., 2011 |
| Human Liver Microsome | To investigate the revers-ible inhibitory or time-dependent inhibitory effects of AS on nine CYP isoforms, including CYP1A2, CYP2A6,CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, andcyp3a4/5, in human liver microsomes. | 0,05, 0,5 or 5 μM ASTX | Astaxanthin implemented on human liver microsome had an weak effect on CYP2C19 and IC50 value was 16,2 μM. | Zheng et al., 2013 |
| Human Neutrophils | To seek connection between glycolaldehyde and human neutrophils | Glycolaldehyde (1 mM) followed or not by the antioxidants astaxanthin (2 mM) and vitamin C (100 mM) for 24 h at 37°C. | Combine application of vitamin C (100 mM) and ASTX (2 mM) decreased output of NO and H2O2 in cells, unfavourable impacts of glycolaldehyde. | Guerra et al., 2012 |
| HeLa human cervical cancer cells, PC12 cells, Jurkat cells |
To investigate effects of ASTX on mitochondrial redox | HeLa cells were cultured in DMEM/F12+10% FBS with or without 800 nM AX (control: DMSO) for 6 h, 1 day and 2 days, then exposed to 30 μg/ml antimycin A for 15 min, then 250 nM MitoSOX Red (Invitrogen) and 500 nM Hoechst34580 (Invitrogen) added and incubated for 60 min, and fluorescence recorded using a 20 × (N.A. 0.5) objective. Astaxanthin effect on PC12 cell survival under oxidative stress. Cells were cultured in the presence of 0, 100, 200 and 400 nM AX for 6 h or 24 h. Jurkat cells cultivated in the presence or absence (DMSO) of AX (800 nM) for 6 h, 1 day, and 2 days were treated with antimycin A (30 μg/ml). |
PC12 cells incubated with 200 and 400 nM ASTX for 24 h were protected against cell death due to oxidative stress. However, 800 nM ASTX had a constructive effect on Jurkat cells. HeLa cell cultured with same dose for 6 h also provided a decrease in H2O2 levels. | Wolf et al., 2010 |
| Human dopaminergic neuroblastoma SH-SY5Y cells | To investigate the effect and the mechanism of astaxanthin on reactive oxygen species (ROS)-mediated apoptosis in dopaminergic SH-SY5Y cells | Human dopaminergic neuroblastoma SH-SY5Y cells were DHAOOH- or 6-OHDA-treated cells. Different concentrations (25, 50, 100, 500, 1000 nM) of astaxanthin were added to the cells for 4 h and 24 h | It was provided a decrease in DHA-OOH levels. ROS production was protected by ASTX in these cells. | Liu et al., 2009 |
| Platelets from C57BL/6 rats | To search effects of pro-drug (CDX-085) on rat platelets | C57BL/6 rats fed 0,4% CDX-085 for 2 weeks that were treated with 1 μM ASTX. | Proinflammatory ONOO- formation was reduced in platelets from C57BL/6 rats fed 0,4% CDX-085 for 2 weeks that were treated with 1 μM ASTX. | Khan et al., 2010 |
Table 3: Cell studies related with ASTX, their procedures and results.
Conclusion
Reactive oxygen species emergent through senescence further expedite the aging process. Several studies that investigated effects of ASTX on oxidative stress suggested that ASTX inhibits oxygen species by impacting enzymes and their pathways. In addition to this, ASTX enhances activation of the antioxidant defence system, which decreases with age. Most of studies on rats and limited availability of human studies cause to be restricted proposal usage of ASTX as diet supplement. Besides this situation, indicated classification diary amounts of ASTX by age may put some teeth into safe intake of ASTX.
Effect and important keystone is also confidential doses of ASTX for both rats and human. It should be paid regard to determine these doses for especially human from the viewpoint of gender, age, health or existence of other diseases, used drugs, genotype and their biochemical values.
Senescence is an inexpugnable sooth. Despite this, reasons leading to it may be intercepted and aging may be postponed by antioxidants, nutritional behaviors and studies. Some cell types used in searches, such as microsome, may not represent real physiology. Of other cruces, procedurs of ASTX and researches pertain to perfect physiology should be done more.
REFERENCES
- Anton B, Vitetta L, Cortizo F, Sali A. Can we delay aging? The biology and science of aging. Ann N Y Acad Sci. 2005;1057(1): 525-535.
- Goldsmith TC. Evolution Theory and Aging. In: Evans E (ed) An Introduction To Biological Aging Theory: Azinet Box 239 Crownville 21032,USA, 2011; Pp. 5-8.
- Lipsky MS, King M. Biological theories of aging. Dis Mon. 2015;61(11): 460-466.
- Medvedev ZA. An attempt at a rational classification of theories of ageing. Biol Rev Camb Philos Soc. 1990;65(3): 375-398.
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3): 298-300.
- Ricklefs RE. The evolution of senescence from a comparative perspective. Funct Ecol. 2008;22(3): 379-392.
- Poljsak B, Milisav I. Aging, oxidative stress and antioxidants. In: Morales-Gonzalez JA (ed) Oxidative Stress and Chronic Degenerative Diseases: A Role for Antioxidants: InTech Janeza Trdine 9, 51000 Rijeka, Croatia, Europe, 2013; pp: 331-353.
- Pae M, Wu D. Nutritional modulation of age-related changes in the immune system and risk of infection. Nutr Res. 2017;41: 14-35.
- Choe E, Min DB. Mechanisms of antioxidants in the oxidation of foods. Compr Rev Food Sci Food Saf. 2009;8(4): 345-358.
- Oroian MA, Escriche I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res Int. 2015;74: 10-36.
- Chapman MS. Vitamin A: History, current uses, and controversies. Paper presented at the seminars in cutaneous medicine and surgery. 2012;31(1): 11-16.
- El-Kholy AA, Elkablawy MA, El-Agamy DS. Lutein mitigates cyclophosphamide induced lung and liver injuryviaNF-κB/MAPK dependent mechanism. Biomed Pharmacother. 2017;92: 519-527.
- Gonçalves AC, Bento C, Silva BM, Silva LR. Sweet cherries from Fundão possess antidiabetic potential and protect human erythrocytes against oxidative damage. Food Res Int. 2017;95: 91-100.
- Li W, Jiang B, Cao X, Xie Y, Huang T. Protective effect of lycopene on fluoride-induced ameloblasts apoptosis and dental fluorosis through oxidative stress-mediated caspase pathways. Chem Biol Interact. 2017;261:27-34.
- Zhang CR, Aldosari SA, Vidyasagar PSPV, Shukla P, Nair MG. Health-benefits of date fruits produced in Saudi Arabia based on in vitro antioxidant, anti-inflammatory and human tumor cell proliferation inhibitory assays. J Saudi Society Agri Sci. 2017;16(3): 287-293.
- Higuera Ciapara I, Felix Valenzuela L, Goycoolea FM. Astaxanthin: A review of its chemistry and applications. Crit Rev Food Sci Nutr. 2006;46(2): 185-196.
- Martínez Delgado AA, Khandual S, Villanueva Rodríguez SJ. Chemical stability of astaxanthin integrated into a food matrix: Effects of food processing and methods for preservation. Food Chem. 2017;225: 23-30.
- Biswal S. Oxidative stress and astaxanthin: The novel supernutrient carotenoid. Int J Health Allied Sci. 2014;3(3): 147-153
- Jyonouchi H, Sun S, Gross M. Effect of carotenoids on in vitro immunoglobulin production by human peripheral blood mononuclear cells: Astaxanthin, a carotenoid without vitamin A activity, enhances in vitro immunoglobulin production in response to a T-dependent stimulant and antigen. Nutr Cancer. 1995; 23(2): 171-183.
- Abdelzaher LA, Imaizumi T, Suzuki T, Tomita K, Takashina M, Hattori Y. Astaxanthin alleviates oxidative stress insults-related derangements in human vascular endothelial cells exposed to glucose fluctuations. Life Sci. 2016;150: 24-31.
- Yang Y, Kim B, Park YK, Koo SI, Lee JY. Astaxanthin prevents TGFbeta1-induced pro-fibrogenic gene expression by inhibiting Smad3 activation in hepatic stellate cells. Biochem Biophys Acta. 2015;1850(1): 178-185.
- Choi HD, Youn YK, Shin WG. Positive effects of astaxanthin on lipid profiles and oxidative stress in overweight subjects. Plant Food Hum Nutr. 2011;66(4):363-369.
- Choi HD, Kim JH, Chang MJ, Kyu-Youn Y, Shin WG. Effects of astaxanthin on oxidative stress in overweight and obese adults. Phytother Res. 2011;25(12): 1813-1818.
- Kim JH, Chang MJ, Choi HD, Youn YK, Kim JT, Oh JM, et al. Protective effects of Haematococcus astaxanthin on oxidative stress in healthy smokers. J Med Food. 2011;14(11): 1469-1475.
- Sila A, Kamoun Z, Ghlissi Z, Makni M, Nasri, M, Sahnoun Z, et al. Ability of natural astaxanthin from shrimp by-products to attenuate liver oxidative stress in diabetic rats. Pharmacol Rep. 2015;67(2): 310-316.
- Baralic I, Djordjevic B, Dikic N, Kotur-Stevuljevic J, Spasic S, Jelic-Ivanovic Z, et al. Effect of astaxanthin supplementation on paraoxonase 1 activities and oxidative stress status in young soccer players. Phytother Res. 2013;27(10): 1536-1542.
- Hussein G, Sankawa U, Goto H, Matsumoto K, Watanabe H. Astaxanthin: A carotenoid with potential in human health and nutrition. J Nat Prod. 2006;69(3): 443-449.
- Arathi BP, Sowmya RP, Vijay K, Baskaran V, Lakshminarayana R. Metabolomics of carotenoids: The challenges and prospects: A review. Trends Food Sci Tech. 2015;45(1): 105-117.
- Wu H, Niu H, Shao A, Wu C, Dixon BJ, Zhang J, et al. Astaxanthin as a potential neuroprotective agent for neurological diseases. Mar Drugs. 2015;13(9): 5750-5766.
- Yang Y, Kim B, Lee JY. Astaxanthin structure, metabolism, and health benefits. J Hum Nutr Food Sci. 2013;1(1003): 1-11.
- Lerfall J. Carotenoids: Occurrence, Properties and Determination. In: Caballero B, Finglas PM, Toldrá F (eds) Encyclopedia of Food and Health, Oxford, Academic Press, UK, 2016; Pp.663-669.
- Coral-Hinostroza GN, Ytrestoyl T, Ruyter B, Bjerkeng B. Plasma appearance of unesterified astaxanthin geometrical E/Z and optical R/S isomers in men given single doses of a mixture of optical 3 and 3'R/S isomers of astaxanthin fatty acyl diesters. Comp Biochem Physiol C Toxicol Pharmacol. 2004;139(1): 99-110.
- Wolz E, Liechti H, Notter B, Oesterhelt G, Kistler A. Characterization of metabolites of astaxanthin in primary cultures of rat hepatocytes. Drug Metab Dispos. 1999;27(4): 456-462.
- FDA. Best Practices for Convening a GRAS Panel: Guidance for Industry: Draft Guidance. U.S. Food and Drug Administration (U.S. FDA), Center for Food Safety & Applied Nutrition (CFSAN), Center for Veterinary Medicine (CVM). 2010 (24.11.2017).
- Harman D. The aging process. Proc Natl Acad Sci. USA 1981;78(11): 7124-7128.
- Vina J, Borras C, Miquel J. Theories of ageing. IUBMB Life. 2008;59(4): 249-254.
- Park DC, Yeo SG. Aging. Korean J Audiol. 2013;17(2): 39-44.
- Croteau DL, Bohr VA. Repair of oxidative damage to nuclear and mitochondrial DNA in mammalian cells. J Biol Chem. 1997;272(41): 25409-25412.
- Harman D. Free radical theory of aging: History. Exs. 1992;62:1-10.
- Meng J, Lv Z, Qiao X, Li X, Li Y, Zhang Y, et al. The decay of redox-stress response capacity is a substantive characteristic of aging: Revising the redox theory of aging. Redox Biol. 2017;11: 365-374.
- Mohammadzadeh A, Mirza-Aghazadeh-Attari M, Hallaj S, Saei AS, Alivand MR, Valizadeh A, et al. Crosstalk between P53 and DNA damage response in ageing. DNA Repair. 2019;80: 8-15.
- Kloska D, Kopacz A, Piechota-Polanczyk A, Nowak WN, Dulak J, Jozkowicz A, et al. Nrf2 in aging: Focus on the cardiovascular system. Vasc Pharmacol. 2019;112: 42-53.
- Barja G. Towards a unified mechanistic theory of aging. Exp Gerontol. 2019;124: 110627.
- Richter C. Oxidative damage to mitochondrial DNA and its relationship to ageing. Int J Biochem Cell Biol. 1995;27(7): 647-653.
- Bowman A, Birch-Machin MA. Age-dependent decrease of mitochondrial complex II activity in human skin fibroblasts. J Invest Dermatol. 2016;136(5):912-919.
- Xu B, Gao Y, Zhan S, Xiong F, Qiu W, Qian X, et al. Quantitative protein profiling of hippocampus during human aging. Neurobiol Aging. 2016;39:46-56.
- Hirose M, Schilf P, Lange F, Mayer J, Reichart G, Maity P, et al. Uncoupling protein 2 protects mice from aging. Mitochondrion. 2016;30: 42-50.
- Tatarkova Z, Kuka S, Racay P, Lehotsky J, Dobrota D, Mistuna D, et al. Effects of aging on activities of mitochondrial electron transport chain complexes and oxidative damage in rat heart. Physiol Res. 2010;60(2): 281-289.
- Du Z,Yang Q, Liu L, Zhao J, Hu J, Liu C, et al. NADPH oxidase 2-dependent oxidative stress, mitochondrial damage and apoptosis in the ventral cochlear nucleus of D-galactose-induced aging rats. Neuroscience. 2015;286: 281-292.
- Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011;301(2): 363-372.
- Meng D, Zhang P, Zhang L, Wang H, Ho CT, Li S, et al. Detection of cellular redox reactions and antioxidant activity assays. J Funct Foods. 2017;37: 467-479.
- Zheng YF, Bae SH, Kwon MJ, Park JB, Choi HD, Shin WG, et al. Inhibitory effects of astaxanthin, β-cryptoxanthin, canthaxanthin, lutein, and zeaxanthin on cytochrome P450 enzyme activities. Food Chem Toxicol. 2013;59: 78-85.
- Elfawy HA, Das B. Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative disease: Etiologies and therapeutic strategies. Life Sci. 2019;218: 165-184.
- Yamashita E. Let astaxanthin be thy medicine. Pharma Nutrition. 2015;3(4): 115-122.
- Kumar SR, Narayan B, Sawada Y, Hosokawa M, Miyashita K. Combined effect of astaxanthin and squalene on oxidative stress in vivo. Mol Cell Biochem. 2016;417(1): 57-65.
- Wu Q, Zhang XS, Wang HD, Zhang X, Yu Q, Li W, et al. Astaxanthin activates nuclear factor erythroid-related factor 2 and the antioxidant responsive element (Nrf2-ARE) pathway in the brain after subarachnoid hemorrhage in rats and attenuates early brain injury. Mar Drugs. 2014;12(12): 6125-6141.
- Xu L, Zhu J, Yin W, Ding X. Astaxanthin improves cognitive deficits from oxidative stress, nitric oxide synthase and inflammation through upregulation of PI3K/Akt in diabetes rat. Int J Clin Exp Pathol. 2015;8(6): 6083-6094.
- Xue Y, Qu Z, Fu J, Zhen J, Wang W, Cai Y, et al. The protective effect of astaxanthin on learning and memory deficits and oxidative stress in a mouse model of repeated cerebral ischemia/reperfusion. Brain Res Bull. 2017;131: 221-228.
- Al-Amin MM, Mahmud W, Pervin MS, Islam SMR, Rahman MA, Zinchenko A. Astaxanthin ameliorates scopolamine-induced spatial memory deficitviareduced cortical-striato-hippocampal oxidative stress. Brain Res. 2019;1710: 74-81.
- Xue XL, Han XD, Li Y, Chu XF, Miao WM, Zhang JL, et al. Astaxanthin attenuates total body irradiation-induced hematopoietic system injury in miceviainhibition of oxidative stress and apoptosis. Stem Cell Res. Ther 2017;8: 7.
- Zhou L, Gao M, Xiao Z, Zhang J, Li X, Wang A. Protective effect of astaxanthin against multiple organ injury in a rat model of sepsis. J Surg Res. 2015;195(2): 559-567.
- Al-Amin MM, Akhter S, Hasan AT, Alam T, Hasan SMN, Saifullah AR, et al. The antioxidant effect of astaxanthin is higher in young mice than aged: A region specific study on brain. Metab Brain Dis. 2015;30(5): 1237-1246.
- Mattei R, Polotow TG, Vardaris CV, Guerra BA, Leite JB, Otton R, et al. Astaxanthin limits fish oil-related oxidative insult in the anterior forebrain of Wistar rats: Putative anxiolytic effects? Pharmacol Biochem Behav. 2011;99(3): 349-355.
- Otsuka T, Shimazawa M, Nakanishi T, Ohno Y, Inoue Y, Tsuruma K, et al. The protective effects of a Dietary Carotenoid, Astaxanthin, against light-induced retinal damage. J Pharm Sci. 2013;123(3): 209-218.
- Kamath BS, Srikanta BM, Dharmesh SM, Sarada R, Ravishankar GK. Ulcer preventive and antioxidative properties of astaxanthin from Haematococcus pluvialis. Eur J Pharmacol. 2008;590(1): 387-395.
- Al-Amin MM, Reza HM, Saadi HM, Mahmud W, Ibrahim AA, Alam MM, et al. Astaxanthin ameliorates aluminum chloride-induced spatial memory impairment and neuronal oxidative stress in mice. Eur J Pharmacol. 2016;777: 60-69.
- Marin DP, Bolin AP, Macedo RCS, Sampaio SC, Otton R. ROS production in neutrophils from alloxan-induced diabetic rats treated in vivo with astaxanthin. Int Immunopharmacol. 2011;11(1): 103-109.
- Pan L, Zhou Y, Li XF, Wan QJ, Yu LH. Preventive treatment of astaxanthin provides neuroprotection through suppression of reactive oxygen species and activation of antioxidant defense pathway after stroke in rats. Brain Res Bull. 2017;130:211-220.
- Kuraji M, Matsuno T, Satoh T. Astaxanthin affects oxidative stress and hyposalivation in aging mice. J Clin Biochem Nutr. 2016;59(2): 79-85.
- Dong LY, Jin J, Lu G, Kang XL. Astaxanthin attenuates the apoptosis of retinal ganglion cells in db/db mice by inhibition of oxidative stress. Marine Drugs. 2013;11(3): 960-974.
- Komaki A, Karimi SA, Salehi I, Sarihi A, Shahidi S, Zarei M. The treatment combination of vitamins E and C and astaxanthin prevents high-fat diet induced memory deficits in rats. Pharmacol Biochem Behav. 2015;131: 98-103.
- Sasaki YN, Kobara S, Higashino JC, Giddings JC, Yamamoto J. Astaxanthin inhibits thrombosis in cerebral vessels of stroke-prone spontaneously hypertensive rats. Nutr Res. 2011;31(10): 784-789.
- Yeh PT, Huang HW, Yang CM, Yang WS, Yang CH. Astaxanthin inhibits expression of retinal oxidative stress and inflammatory mediators in streptozotocin-induced diabetic rats. PLoS One. 2016;11(1): e0146438.
- Masoudi A, Dargahi L, Abbaszadeh F, Pourgholami MH, Asgari A, Manoochehri M, et al. Neuroprotective effects of astaxanthin in a rat model of spinal cord injury. Behav Brain Res. 2017;329: 104-110.
- Wu W, Wang X, Xiang Q, Meng X, Peng Y, Du N, et al. Astaxanthin alleviates brain aging in rats by attenuating oxidative stress and increasing BDNF levels. Food Funct. 2014;5(1): 158-166.
- Fakhri S, Dargahi L, Abbaszadeh F, Jorjani M. Astaxanthin attenuates neuroinflammation contributed to the neuropathic pain and motor dysfunction following compression spinal cord injury. Brain Res Bull. 2018;143: 217-224.
- Rahman SO, Panda BP, Parvez S, Kaundal M, Hussain S, Akhtar M, et al. Neuroprotective role of astaxanthin in hippocampal insulin resistance induced by Aβ peptides in animal model of Alzheimer’s disease. Biomed Pharmacother. 2019;110: 47-58.
- Fanaee Danesh E, Gali CC, Tadic J, Zandl Lang M, Kober AC, Agujetas VR, et al. Astaxanthin exerts protective effects similar to bexarotene in Alzheimer's disease by modulating amyloid-beta and cholesterol homeostasis in blood-brain barrier endothelial cells. Biochim Biophys Acta Mol Basis Dis. 2019;1865(9): 2224-2245.
- Yamagishi R, Aihara M. Neuroprotective effect of astaxanthin against rat retinal ganglion cell death under various stresses that induce apoptosis and necrosis. Mol Vis. 2014;20: 1796-1805.
- Farruggia C, Kim MB, Bae M, Lee Y, Pham T, Yang Y, et al. Astaxanthin exerts anti-inflammatory and antioxidant effects in macrophages in NRF2-dependent and independent manners. J Nutr Biochem. 2018;62: 202-209.
- Wen X, Huang A, Hu J, Zhong Z, Liu Y, Li Z, et al. Neuroprotective effect of astaxanthin against glutamate-induced cytotoxicity in HT22 cells: Involvement of the Akt/GSK-3beta pathway. Neuroscience. 2015;303: 558-568.
- Li Z, Dong X, Liu H, Chen X, Shi H, Fan Y, et al. Astaxanthin protects ARPE-19 cells from oxidative stressviaupregulation of Nrf2-regulated phase II enzymes through activation of PI3K/Akt. Mol Vis 2013;19: 1656-1666.
- Guerra BA, Bolin AP, Morandi AC, Otton R. Glycolaldehyde impairs neutrophil biochemical parameters by an oxidative and calcium-dependent mechanism: Protective role of antioxidants astaxanthin and vitamin C. Diabetes Res Clin Pract. 2012;98(1): 108-118.
- Wolf AM, Asoh S, Hiranuma H, Ohsawa I, Iio K, Satou A, et al. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J Nutr Biochem. 2010;21(5): 381-389.
- Liu X, Shibata T, Hisaka S, Osawa T. Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cellsviamitochondria-targeted protective mechanism. Brain Res. 2009;1254: 18-27.
- Khan SK, Malinski T, Mason RP, Kubant R, Jacob RF, Fujioka K, et al. Novel astaxanthin prodrug (CDX-085) attenuates thrombosis in a mouse model. Thromb Res. 2010;126(4): 299-305.
- Campoio TR, Oliveira FA, Otton R. Oxidative stress in human lymphocytes treated with fatty acid mixture: Role of carotenoid astaxanthin. Toxicol in vitro. 2011;25(7): 1448-1456.
- Franceschelli S, Pesce M, Ferrone A, De Lutiis MA, Patruno A, Grilli A, et al. Astaxanthin treatment confers protection against oxidative stress in U937 cells stimulated with lipopolysaccharide reducing O2- production. PLoS One. 2014;9(2): e88359.
- Zhang Y, Unnikrishnan A, Deepa SS, Liu Y, Li Y, Ikeno Y, et al. A new role for oxidative stress in aging: The accelerated aging phenotype in Sod1(−/)(−) mice is correlated to increased cellular senescence. Redox Biol. 2017;11: 30-37.
Citation: Deveci G, Tek NA (2019) A Mini Review of Astaxhantin and Oxidative Stress in Aging. J Aging Sci. 7: 211. Doi: 10.35248/2329-8847.19.07.211.
Copyright: © 2019 Deveci G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.