PMC/PubMed Indexed Articles
Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 12, Issue 1
A Full Replicate in vivo Bioequivalence Study of Two Idelalisib 150 mg Tablets in Fasted Healthy Adult Human Subjects
Arjun Arumugam1*, Aparna Mani1 and Juan Chirinos22Abbott Laboratories de Colombia, Colombia
Received: 20-Sep-2019 Published: 08-Jan-2020, DOI: 10.35248/0975-0851.20.12.391
Abstract
Background: Idelalisib, a PI3K small molecule inhibitor, specifically blocks the phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit delta isoform (PI3Kδ). This is a potent drug which is specifically targeted for relapsed chronic lymphocytic leukemia (CLL).
Methods and Findings: A full replicate bioequivalence study of two Idelalisib 150 mg tablets was conducted in 56 healthy adult human subjects under fasting conditions with a washout period of 10 days in between doses. Blood samples were collected up to 72 hours post-dose for measurement of pharmacokinetic parameters in all periods to quantify Idelalisib in human plasma using a validated LC-MS/MS method. Bioequivalence between both the products was established by calculating 90% confidence intervals (90% CI) for the ratio of Cmax and AUC0-t values for the test and reference products. The 90% confidence intervals found for the relation of Test/Reference were Cmax 92.23% - 106.06% and AUC0-t 96.62% - 105.27%.
Conclusion: According to FDA's guidelines for Bioequivalence research and based on the ANOVA results obtained, it can be concluded that Idelalisib 150 mg tablets of Abbott Laboratories de Colombia is bioequivalent to Zydelig (Idelalisib) 150 mg tablets of Gilead Sciences Ltd under fasting conditions.
Keywords
Bioequivalence; Fully replicate bioequivalence; Healthy subjects; Idelalisib
Introduction
Idelalisib is the first approved drug by FDA which is the inhibitor of PI3Kδ kinase. These enzymes are responsible for regulating various cellular functions. The drug induces apoptosis and inhibits proliferation in cell lines derived from malignant B cells and in primary tumor cells. Idelalisib inhibits several cell signaling pathways, including B-cell receptor (BCR) signaling and CXCchemokine receptor (CXCR)4 and CXCR5 signaling, which are involved in trafficking and homing of B cells to the lymph nodes and bone marrow [1].
Idelalisib is a highly selective oral inhibitor of p110δ which in combination with anti-CD20 monoclonal antibodies is said to be a potent drug for the treatment of relapsed/refractory chronic lymphocytic leukemia. Idelalisib is metabolized via aldehyde oxidase and CYP3A to an inactive metabolite [2].
The drug can be administered orally with or without food. It undergoes hepatic metabolism leading to a metabolite [3]. Post administration of the drug as a single dose orally, the peak plasma concentration profile were observed to be 0.5 to 1.5 hours under fasted conditions and 2 to 4 hours under fed conditions.
When the drug is administered in capsule formulation with a high-fat high-calorie meal, there were no changes observed in Cmax but AUCinf was observed to be increased by 36%. Idelalisib can be administered without food. Idelalisib is 93% to 94% bound to human plasma proteins at concentrations observed clinically.
The terminal elimination half-life of Idelalisib was 8.2 hours. Following a single 150 mg oral dose of [14C]-labelled Idelalisib, approximately 78% and 15% was excreted in faeces and urine, respectively.
As required by various health authorities around the world, bioequivalence for this product is required to be evaluated in fasting conditions, per the product label [4-6].
A generic version of Idelalisib 150 mg tablets were developed by the sponsor of this study and the current study was planned to evaluate its bioequivalence; Based on available information on the pharmacokinetic parameters, the drug product was expected to demonstrate high within subject variability [7].
Thus, in accordance with EMA’s current guidance on bioequivalence [7], a fully replicate design was planned for the bioequivalence study, which allowed to establish the within subject variability and expand the 90% confidence interval limits for BE based on the same. Bioequivalence studies provides interchangeability between generic products and reference products without reiterating clinical trials in patients [6].
Objective of the study was to assess the bioequivalence of Idelalisib 150 mg tablets of Abbott Laboratories de Colombia and Zydelig (Idelalisib) 150 mg tablets of Gilead Sciences Ltd in healthy, adult, human subjects under fasting conditions.
Methods
Inclusion criteria
Healthy males and/or non-pregnant, non-lactating female literate volunteers of 20 to 45 years (both years inclusive) with BMI of 18.50 – 29.99 Kg/m2 and weight > 50kg were enrolled. Healthy volunteers as evaluated by medical history, vitals and general clinical examination, normal or clinically insignificant biochemical, hematological, urinary, serology and other safety parameters were enrolled. Negative urine test for drugs of abuse, alcohol breath analysis for both males and females and negative pregnancy tests for females and also willingness to practice birth control were enrolled. Female subjects who are willing to practice acceptable methods of contraception and volunteers who can give written informed consent and communicate effectively were enrolled in the study.
Exclusion criteria
Volunteers with a history of any major surgical procedure in the past 3 months, any clinically significant cardiac, gastrointestinal, respiratory, hepatic, renal, endocrine, neurological, metabolic, psychiatric, hematological disorders were not enrolled. Volunteers with a history of chronic alcoholism/chronic smoking/drug of abuse and subject who consumed tobacco containing products within 48 hours prior to proposed time of dosing were not considered to participate in the study. Present or past history of intake of drugs or any prescription drug or over the counter (OTC) drugs within 14 days which potentially modify kinetics / dynamics of Idelalisib or any other medication judged to be clinically significant by the investigator were not enrolled. Consumption of grapefruit and/or its products within 10 days prior to the start of study and subjects who had participated in any other clinical study or who had bled during the last 3 months before check-in subjects who consumed one or more of the below, 48 hours prior to dosing: Xanthine containing food or drinks such as cola, coffee or tea, citrus fruits or items (lime, lemon and orange), alcohol and any other food/beverage known to have interactions as deemed by the investigator were not enrolled. Volunteers who are dysphagic were not considered for participate in the study.
Removal of subjects from therapy or assessment
Subject willing to withdraw consent for any reason, development of intolerable adverse event, subjects who do not follow study restrictions and subjects who are non-cooperative, per the discretion of the investigator and subjects who requires administration of medicines that are known to interfere with the pharmacokinetics of Idelalisib were considered as withdrawal criteria for this study.
Informed consent
The protocol and informed consent were reviewed and approved by the Institutional Review Board (IRB): “Chennai Meenakshi Multispecialty Hospital Ethics Committee”, (Ethics Committee re-registration No. ECR/516/lnst/TN/2014/RR-17 issued under Rule 12200 of the Drugs & Cosmetics Rules, Ministty ofHealth & Family Welfare, Directorate General of Health Services, Office of the Drugs Controller General (India), Central Drugs Standard Control Organization). Written informed consent was obtained from each volunteer for screening prior to initiation of screening procedure and for the study prior to enrolment. The study informed consent documents were distributed to the eligible subjects. The consent documents were read and explained by the staff. Subjects were given adequate time to read and understand the consent documents. Individual counseling was then given to the willing volunteers by the Investigator in private and any questions and concerns were addressed prior to obtaining consent.
The study was conducted as per the ICMR Ethical Guidelines for Biomedical Research on Human Subjects (2016), Schedule –Y of Drugs and Cosmetic Acts, 20th January 2005 and its amendments, ICH-GCP and in compliance with the ethical principles enunciated in the Declaration of Helsinki-2013 [7,8].
Study design
An open label, randomized, two treatment, two sequence, four period, single dose, fully replicate, cross over, oral bioequivalence study of Idelalisib 150 mg tablets of Abbott Laboratories de Colombia and Zydelig (Idelalisib) 150 mg tablets of Gilead Sciences Ltd in healthy, adult, human subjects under fasting conditions. Study subjects received either test or reference in each period as per the randomization schedule. The randomization schedule was generated by using SAS® software and each study subject was randomly assigned to one of the sequences of test product (T) and reference product (R) for the study. Schematic representation of the study design is shown in Figure 1.
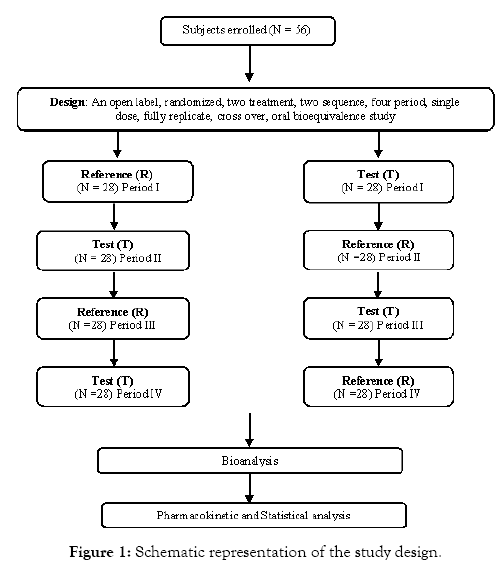
Figure 1: Schematic representation of the study design.
Drug administration
After an overnight fasting of 8 hours, the subjects were administered with a single oral dose of either test product or reference product with 200 mL of water as per the randomization schedule in sitting posture at ambient temperature in each period. Washout period of 10 days was maintained between the treatments [9].
Dosing was performed by trained personnel according to the randomization scheme under the supervision of Investigators/ Quality Control/Quality Assurance personnel. Compliance to dosing following drug administration was assessed by examination of the oral cavity. Drug dosing details were recorded in the individual case report forms. The Principal Investigator and/or Sub investigator/Physician was present throughout the conduct of the study.
All the subjects remained in upright posture for 04 hours post dosing and were permitted to walk for short intervals of time for reasons such as but not limited to the following - natural exigencies, blood sampling and vitals measurement activity. Water restriction of 01 hour prior to and post dose was followed as per the protocol by all the subjects and were allowed to consume water ad libitum thereafter. The investigational products for dosing were dispensed in pharmacy on a working day prior to drug administration. Stand-by for both test and reference formulations were also dispensed to replace for any damaged or spilled drug. Details of the investigational products are given in Table 1.
| Test product | Idelalisib 150 mg tablets of Abbott Laboratories de Colombia |
| Reference product | Zydelig (Idelalisib) 150 mg tablets of Gilead Sciences Ltd. |
Table 1: Investigational products.
Blood sampling
A total of 19 blood samples, 05 mL each at 00.00 hour (predose), 00.25, 00.50, 00.75, 01.00, 01.50, 02.00, 02.50, 03.00, 03.50, 04.00, 04.50, 05.00, 06.00, 08.00, 12.00, 24.00, 48.00 and 72.00 hours post dose were collected for measurement of pharmacokinetic parameters in all the four periods. The pre dose samples were collected 02 hours prior to the drug administration. The post dose in-house samples were collected within 02 minutes from the scheduled time and the ambulatory samples were collected within ± 02 hours from the schedule time. Any delay from these was considered as a protocol deviation [10].
The samples were collected from intravenous cannula or by direct vein puncture into pre- labeled K3EDTA vacutainers. The samples were then centrifuged at 4000 rpm for 10 minutes at 4°C. The plasma was separated into two aliquot: 01 mL in first aliquot and remaining in the second aliquot. The K3EDTA vacutainers and polypropylene tubes were pre-labeled with Subject Number, Study Number, Study Period, Time point and Aliquot Number. The samples were stored at -70°C ± 15°C in an ultra-low temperature freezer pending segregation and shipment. The plasma samples were quantified using validated bio-analytical method.
Analytical method
Solid Phase Extraction was chosen for sample preparation to extract the analyte of Idelalisib. A validated LC-MS/MS bioanalytical method was used for estimation of Idelalisib in plasma. Bioanalytical method validation was done as per FDA’s Bioanalytical Method Validation guidance on Specificity, Sensitivity, Precision and Accuracy, Stability, Recovery, Dilution Integrity and Linearity range. Samples of subjects who completed the entire duration of study were analysed. All samples from one subject were analyzed with the same single standard curve. Sample concentration above upper limit of the standard curve from validated range was analysed by diluting the sample with drug free biological matrix and assayed. A calibration curve range of 70.059 to 7017.540 ng/ml was used and was found to be acceptable for application.
Pharmacokinetic parameters and statistical analysis
Pharmacokinetic parameters were calculated using Phoenix® WinNolin software (version 7.0). The mean, standard deviation, standard error, geometric mean, coefficient of variation, minimum, median, maximum and range were calculated for AUC0-t and Cmax. All concentrations below the level of quantification (BLQ) were set to zero (0) for pharmacokinetic calculations and statistical analysis.
The analysis of variance (ANOVA) was performed on the Lntransformed data of AUC0-t and Cmax, which included sequence, period and formulation (treatment) as fixed effect and the subjects nested within the sequence as random effect. The sequence effect was tested at the 0.10 level of significance using the subjects nested within sequence mean square from the ANOVA as the error term. All other main effects were tested at the 0.05 level of significance against the residual error (mean square error/MSE) from the ANOVA as the error term using 90% confidence interval approach [8]. Based on comparisons of the test and reference product for Ln-transformed AUC0-t and Cmax data, the ratio of the least square mean was calculated, as well as the 90% confidence intervals for Ln-transformed AUC0-t and Cmax of Idelalisib for test and reference products was determined.
Safety analysis
Safety was assessed via continuous health monitoring and scheduled recording of safety measurements throughout the study. Staff monitored and recorded the pulse rate, blood pressure and subjects’ well-being during check-in, at 00.00 (pre dose), 02.00, 08.00, and 23.00 hours post-dose in each period, during ambulatory, post study and at discretion of clinical staff. Temperature was recorded during check in, prior to check out and post study. The pre dose (00.00 hour) vital parameters were recorded within two hours prior to drug administration and the post dose vitals with a flexibility of ± 30 minutes of each time point. During ambulatory visits, the vital parameters were recorded at the actual time of visit, before blood sampling.
General examination and systemic examination of subjects were performed during check-in of each period by the physician/ investigator/sub-investigator to ensure safety. Subjects were instructed to inform clinic personnel of any untoward medical symptoms and/or events that arose during the course of the study. Prior to check in of each period, subjects were questioned concerning unusual symptoms that may have occurred after the previous administration of the study drug. The Principal Investigator/sub-investigator/study physician evaluated the relationship of all adverse events to the study drugs.
Results
Demographic characteristics
The mean age, height, weight and BMI of the 53 subjects who completed the study are presented in Table 2. All the subjects included in the study were Male and Asians.
| Parameters | Mean | SD | Min | Max |
|---|---|---|---|---|
| Age (years) | 32 | 6 | 24 | 41 |
| Height (m) | 1.665 | 0.053 | 1.500 | 1.800 |
| Weight (Kg) | 67.4 | 9.6 | 50.8 | 86.7 |
| BMI (Kg/m2) | 24.26 | 3.00 | 18.64 | 29.41 |
Table 2: Summarized demographic profile of subjects who completed the study.
Pharmacokinetics and statistics
Out of 56 subjects, 53 subjects completed the study and 03 subjects dropped out from the study. 02 subjects dropped out due to adverse event and 01 subject did not turn up to the facility in period II. Thus, the pharmacokinetic and statistical analysis of Idelalisib was performed using the concentration data obtained from 53 subjects. The plasma concentration vs. time curve of test and reference in fasting conditions is presented in Figure 2. The Geometric mean ratios, 90% CI, power and intra-subject coefficient of variation of test and references for Ln transformed pharmacokinetic parameters Cmax and AUC0-t for Idelalisib are presented in Table 3.
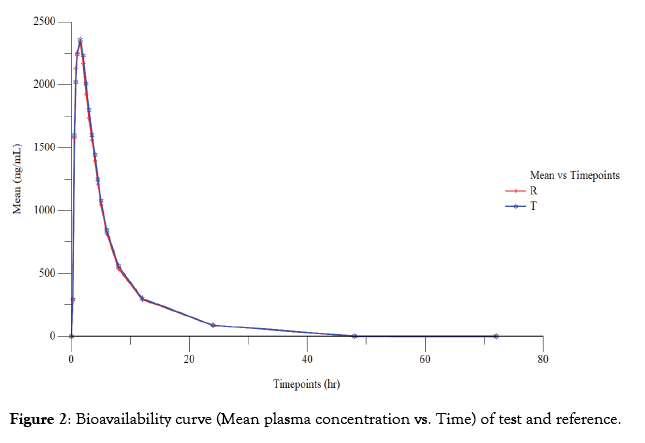
Figure 2: Bioavailability curve (Mean plasma concentration vs. Time) of test and reference.
| Parameters | Antilog Least Square Mean | Point Estimate (%) | 90% Confidence Interval | Intra subject CV (%) | Intra subject CV (%) R vs. R | Power (%) | |
|---|---|---|---|---|---|---|---|
| Test Product (T) | Reference Product (R) | ||||||
| Ln (Cmax ) | 2555.4462 | 2583.7978 | 98.90 | 92.23%-106.06% | 31.47 | 34.06 | 99.98 |
| Ln (AUC0-t) | 13509.5448 | 13395.0116 | 100.86 | 96.62%-105.27% | 19.03 | 20.62 | 100.00 |
Table 3: The Geometric mean ratios, 90% CIs, power and intra subject coefficient of variation of test and reference for Ln transformed pharmacokinetic parameters Cmax and AUC0-t for Idelalisib are presented below.
Safety
Brief summary of adverse events: Safety was evaluated throughout the study and there were 11 adverse events experienced by 06 subjects during conduct of the study. No serious adverse events were reported during the conduct of this study. Among subjects who were administered with reference product, 07 AEs were associated with 04 subjects and among subjects who were administered with test product, 03 AEs were associated with 02 subjects. All the adverse events were resolved without any sequelae. Thus, both the test and reference products can be concluded to be tolerated by subjects. List of adverse events with relation to the drug product – test, reference and post study evaluation are represented in Tables 4, 5 and 6 respectively.
| Subject # | Preferred Terminology (PT) | Number of events | Intensity | Relationship to the drug product |
|---|---|---|---|---|
| S02 | Pruritus | 01 | Moderate | Probable |
| S19 | Pruritus | 01 | Moderate | Probable |
| S02 | Chest pain | 01 | Mild | Unlikely |
| S19 | Rash | 01 | Moderate | Probable |
| S26 | Pyrexia | 01 | Moderate | Probable |
| Headache | 01 | Moderate | Probable | |
| S53 | Rhinitis | 01 | Moderate | Unlikely |
Table 4: List of adverse events with relation to the drug product – Reference.
| Subject | Preferred Terminology (PT) | Number of events | Intensity | Relationship to the drug product |
|---|---|---|---|---|
| S26 | Abdominal pain | 01 | Moderate | Probable |
| Vomiting | 01 | Mild | Probable | |
| S50 | Pyrexia | 01 | Moderate | Probable |
Table 5: List of adverse events with relation to the drug product – Test.
| Subject # | Preferred Terminology (PT) | Number of events | Intensity | Relationship to the drug product |
|---|---|---|---|---|
| S49 | Liver function test increased | 01 | Mild | Probable |
Table 6: List of adverse events with relation to the drug product -Post study evaluation.
Discussion
56 subjects in the age group of 24 to 41 years, who met the study eligibility criteria, participated in the study and 53 subjects completed the study. The clinical study was conducted over a period of 36 days. Blood sampling was done at pre-defined intervals up to 72.00 hours in all periods, separated by a washout period of 10 days between each period. The plasma concentrations of Idelalisib were measured for 53 subjects.
The pharmacokinetic and statistical analyses of Idelalisib were performed using the concentration data obtained from 53 subjects.
The results of the pharmacokinetic analysis of Idelalisib with the test product were comparable to the reference product.
The ISCV of reference product for Cmax is 34.06% and the 90% confidence interval of the relative mean Cmax of the test to reference drug product for Ln-transformed data is within the widened limits of 77.74% to 128.64% based on the ISCV obtained for the reference product. As per prior data available, such high intra subject variability was observed and thus a full replication was considered in the study designing, which allows for expandable BE limits. Although the ISCV was over 30% and allowed for an expandable BE limit, we note, here the BE limits were within 80- 125% for the 90% confidence interval, owing to adequate sample size and high comparability of the Test and Reference products.
Conclusion
Based on the results obtained, it can be concluded that Idelalisib 150 mg tablets of Abbott Laboratories de Colombia is bioequivalent to Zydelig (Idelalisib) 150 mg tablets of Gilead Sciences Ltd in healthy, adult, human subjects under fasting conditions. High variability is noted in the pharmacokinetic parameters of the current study and thus warrants the use of a full replicate design when considering future bioequivalence studies on Idelalisib 150 mg tablets.
Conflict of Interest
This scientific article was made with funding from Abbott Laboratories of Colombia, whose participation was related to financial support and document review. All technical, clinical and analytical execution of the bioequivalence study was performed independently by Azidus Laboratories LTD.
REFERENCES
- Gerson SL, Caimi PF, William BM, Creger RJ. Pharmacology and molecular mechanisms of antineoplastic agents for hematologic malignancies. Hematology. 2018; p: 849-912.
- Lampson BL, Kasar SN, Matos TR, Morgan EA, Rassenti L, Davids MS, et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood. 2016;128(2):195-203.
- Khan M, Saif A, Sandler S, Mirrakhimov AE. Idelalisib for the treatment of chronic lymphocytic leukemia. ISRN oncology. 2014;Pp:1-7.
- https://www.medicines.org.uk/emc/product/3329/smpc
- https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206545Orig1s000ClinPharmR.pdf
- Perry R. Perspectives on the bioequivalence and therapeutic equivalence of generic formulations: An overview of the landscape. Clin Ther. 2010;32(10):1796-1797.
- Committee for Medicinal Products for Human Use. EMEA Guideline on the investigation of bioequivalence. London 20 January 2010. Doc Ref.
- World Medical Association. Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. Adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, and amended by the 59th WMA General Assembly, Seoul, and October 2008.
- Singh J. International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. J Pharmacol Pharmacother. 2015;6(3):185.
- Food US. Drug Administration Center for Drug Evaluation and Research: Guidance for industry: statistical approaches to establishing bioequivalence. Washington, DC: US Department of Health and Human Services. Food and Drug Administration Center for Drug Evaluation and Research. 2001.
Citation: Arumugam A, Mani A, Chirinos J (2019) A Full Replicate in vivo Bioequivalence Study of Two Idelalisib 150 mg Tablets in Fasted Healthy A dult Human Subjects. J B ioequiv Availab 12:391. doi: 1 0.35248/0975-0851.20.12.391
Copyright: © 2019 Arumugam A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


