Indexed In
- Open J Gate
- JournalTOCs
- The Global Impact Factor (GIF)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 9, Issue 1
A Disproportionality Analysis of the Risk of Rhabdomyolysis Associated with DPP-4 Inhibitors in the Food and Drug Administration Adverse Event Reporting System
Wenhui Shi*, Lei Ba and Zhiming SunReceived: 15-Oct-2020 Published: 29-Nov-2020, DOI: 10.35248/2329-6887.20.8.295
Abstract
Backgrounds: Dipeptidyl peptidase 4 inhibitors (DPP4is) are widely used in patients with type 2 diabetes mellitus. Recently safety report from regulatory agency suggested DPP4is may be associated with rhabdomyolysis, thus we performed a detailed analysis and evaluated the association between DPP4is and rhabdomyolysis in the Food and Drug Administration Adverse Event Reporting System (FAERS).
Methods: We examined the FAERS database from 2004q1 to 2017q3 (for a total of 9,906,642 reports), calculated the rates of rhabdomyolysis within the reports for DPP4is and reports for other drugs. After filtering concomitant drugs, we compared proportional reporting ratios (PRRs) among adverse events (AE) reports that listed DPP4is with and without these moderator drugs, to identify whether rhabdomyolysis is associated with the use of DPP4is alone.
Results: 536 rhabdomyolysis AE reports involving DPP4is and 28462 reports involving other drugs were retrieved, the crude PRR for rhabdomyolysis associated with DPP4is was 2.06 (95%CI: 1.89-2.24). After filtering the moderator drugs, the PRR was 2.49 (95%CI: 2.08-2.98). Subanalysis showed PRR of alogliptin (11.89, 95%CI: 6.77-20.87) was higher than other gliptins and PRRs in elderly people were higher than that in working age population, regarding male or female.
Conclusions: Based on this pharmacovigilance analysis, DPP4is may associated with rhabdomyolysis independently, especially alogliptin. DPP4is associated rhabdomyolysis are more likely happened on elderly people which needed to be noted in clinical practice.
Keywords
Glucose-lowering drug; DPP4 inhibitor; Pharmacovigilance; Rhabdomyolysis; Database research
Abbrevations
AE; Adverse event
ARBs; Angiotensin II receptor blockers
CYP; Cytochrome P450
DPP4is; Dipeptidyl peptidase 4 inhibitors
FAERS; Food and Drug Administration Adverse Event Reporting System
FDA; Food and Drug Administration
GLP-1; Glucagon-like-peptide 1
HMG-CoA; 3-hydroxy-3-methyl glutaryl coenzyme A
ICH E2B; International Conference on Harmonisation
MedDRA; Medical Dictionary for Regulatory Activities
PRR; Proportional reporting ratio
T2DM; Type 2 diabetes mellitus
Introduction
Dipeptidyl peptidase 4 inhibitors (DPP4is), also referred to as gliptins, are widely used in many countries as glucose-lowering drugs for patients with type 2 diabetes mellitus (T2DM) [1]. These agents improve glycemic control through suppressing the inactivation of glucagon-like-peptide 1 (GLP-1), leading to reduced glucagon release and increased β-cell survival [2]. Since sitagliptin, the first DPP4is approved by US Food and Drug Administration (FDA) in October 2006 [3], the excellent tolerability/safety profile of DPP4is is supported by published clinical trials [4,5]. However, post marketing experience has raised some concerns, such as potential increase in the risk of pancreatitis, heart failure and hypersensitivity reactions [6].
Recently, FDA quarterly safety report which issued in early October 2017 stated that DPP4is may be associated with rhabdomyolysis [7], a severe syndrome of muscle injury associated with myoglobinuria, electrolyte abnormalities, and often leads to acute kidney injury. In addition, several cases reported rhabdomyolysis associated with combination use of sitagliptin and statins [8-10], as rhabdomyolysis is a known adverse effect of statins and lipid-modifying drugs are commonly used in patients with diabetes, it is necessary to investigate the association between DPP4is and rhabdomyolysis by excluding effects of statins and other co-used drugs.
Many drugs can cause myopathies and may result in rhabdomyolysis such as rheumatologic and immunosuppressive drugs, antiinfectious drugs, lipid-lowering agents, gastrointestinal drugs and etc. [11]. Given that many of abovementioned drugs may be coused with DPP4is in diabetic patients because cardiovascular disease are common complications in the natural history of diabetes, considering moderation effect by drug-drug interaction is necessary.
To this end, in the present study, we analyzed the disproportional association between DPP4is and rhabdomyolysis in FDA Adverse Events Reporting system (FAERS), a global spontaneous reporting database, specifically focusing on potential drug-drug interactions, aimed to complement information from observational case reports and provide further guidance for clinicians.
Methods
FAERS database stores millions of adverse events (AE) reports which contain the demographic characteristics of the patients, drug exposures, indications for use, as well as types and outcomes of reactions [12]. FAERS is a useful tool for looking for new safety signals that might be related to a marketed product, in pharmacovigilance, when certain AE for a given drug are being reported with a greater frequency than would be expected by chance based on a statistical model, we say a safety signal may have been detected.
We used AERSmine [13], a web-based software to mine the FAERS database from 2004q1 to 2017q3, for a total of 9,906,642 reports. This tool normalized and unified drug entries to known generic names, categorized into therapeutic classes based on the Anatomical Therapeutic Chemical (ATC) nomenclature. Moreover, it aggregated clinical indications and adverse events according to Medical Dictionary for Regulatory Activities (MedDRA) Ontology (version 16.1). All the work made user-driven selection and partition across the FAERS data elements possible.
We searched all rhabdomyolysis reports in which DPP4is was used as suspect or concomitant drugs (See electronic supplementary material [ESM] Methods) to access the number of cases, indications and concomitant drugs, to calculate the rates and proportional reporting ratios (PRRs). Then we listed all drugs which were confirmed to be relevant with rhabdomyolysis in the above retrieved DPP4is-rhabdomyolysis reports, performed disproportionality analyses based upon the case/noncase approach [14], computed the PRRs for each drug combination (no DPP4is+no moderator drugs,
DPP4is+no moderator drugs, moderator drugs+no DPP4is, DPP4is+moderator drugs). According to the interaction additive model [15], when the PRR value for co-exposure exceeds the sum of the PRRs for each individual drug, a potential drug-drug interaction may exist.
In subanalysis, we performed same analysis on subgroups that classified by patients’ age, sex [working age (15-65 years) male, working age female, elderly (>65years) male and elderly female] and types of DPP4is (alogliptin, linagliptin, saxagliptin, sitagliptin and vildagliptin). Figure 1 illustrates the study query flow chart.
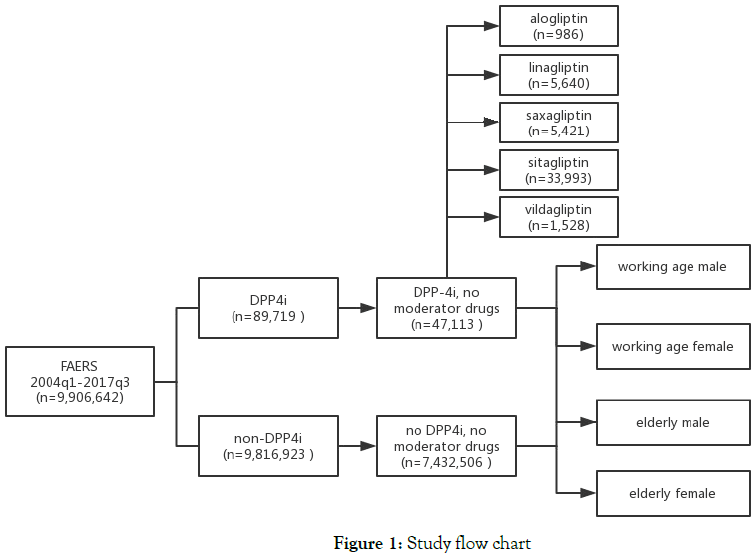
Figure 1. Study flow chart
Statistical Analysis
For each search query, we fetched the total number of rhabdomyolysis AE reports, the respective frequencies/1000 reports and calculated PRRs with 95% CI. Statistical significance was accepted at P<0.05.
Results
Rhabdomyolysis rates in reports field for DPP4is and non- DPP4is drugs
From 2004q1 to 2017q3, there were 89,719 AE reports listing DPP4is as suspect or concomitant drugs (0.91% of FAERS reports). Among these, 536 included rhabdomyolysis as an AE, the rate was 5.97/1000 reports. Among 9,816,923 reports listing any non-DPP4is as suspect or concomitant drugs, 28462 included at least one rhabdomyolysis AE, equal to a rate of 2.90/1000. As a result, the PRR for rhabdomyolysis associated with DPP4is was 2.06 (95%CI: 1.89-2.24).
Among 536 rhabdomyolysis reports with DPP4is also listing 60 other drugs categorized by 8 system organ class (ESM Table 1), which have rhabdomyolysis as a known drug reaction. As expected, the most common moderator drugs were statins and angiotensin receptor blockers, field for cardiovascular system (Figure 2).
| Drugs Class | Drugs | Counts |
|---|---|---|
| alimentary tract and metabolism | rabeprazole | 14 |
| pantoprazole | 32 | |
| sulfasalazine | 1 | |
| antiinfectives for systemic use | ciprofloxacin | 14 |
| benzylpenicillin | 3 | |
| sulfamethoxazole | 7 | |
| cefdinir | 1 | |
| daptomycin | 5 | |
| efavirenz | 2 | |
| lamivudine | 3 | |
| levofloxacin | 3 | |
| clarithromycin | 3 | |
| fusidic acid | 2 | |
| tenofovir disoproxil | 1 | |
| antineoplastic and immunomodulating agents | ciclosporin | 8 |
| sunitinib | 2 | |
| cytarabine | 1 | |
| cardiovascular system | valsartan | 55 |
| olmesartan medoxomil | 31 | |
| hydrochlorothiazide | 73 | |
| pitavastatin | 14 | |
| rosuvastatin | 82 | |
| irbesartan | 21 | |
| losartan | 26 | |
| atorvastatin | 123 | |
| irbesartan and diuretics | 9 | |
| ezetimibe | 48 | |
| amiodarone | 21 | |
| candesartan | 23 | |
| simvastatin | 115 | |
| aliskiren | 4 | |
| olmesartan medoxomil and diuretics | 5 | |
| bezafibrate | 7 | |
| lovastatin | 10 | |
| telmisartan | 9 | |
| fenofibrate | 13 | |
| tolvaptan | 2 | |
| pravastatin | 9 | |
| gemfibrozil | 8 | |
| indapamide | 4 | |
| fluvastatin | 5 | |
| nicotinic acid | 3 | |
| dermatologicals | terbinafine | 2 |
| musculo-skeletal system | tizanidine | 8 |
| colchicine | 11 | |
| febuxostat | 6 | |
| nervous system | pregabalin | 20 |
| oxycodone | 10 | |
| risperidone | 12 | |
| temazepam | 3 | |
| citalopram | 5 | |
| escitalopram | 4 | |
| bupropion | 3 | |
| pramipexole | 2 | |
| aripiprazole | 3 | |
| venlafaxine | 2 | |
| olanzapine | 2 | |
| quetiapine | 2 | |
| propofol | 1 | |
| respiratory system | theophylline | 7 |
ESM Table 1: Other drugs listed in 536 DPP4is associated rhabdomyolysis reports, which have rhabdomyolysis as a known drug reaction.
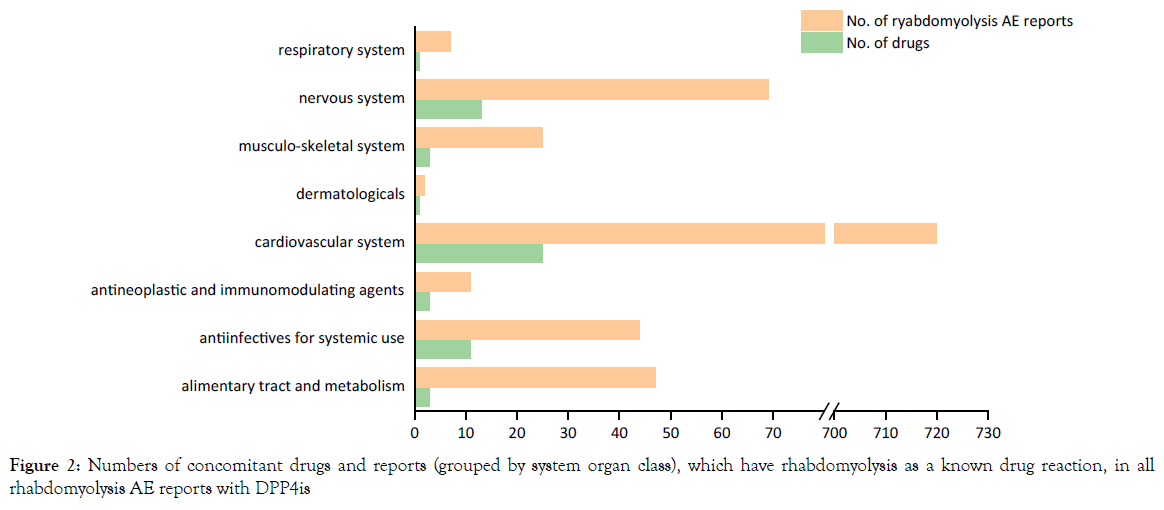
Figure 2. Numbers of concomitant drugs and reports (grouped by system organ class), which have rhabdomyolysis as a known drug reaction, in all rhabdomyolysis AE reports with DPP4is
Moderation of rhabdomyolysis rates by drug-drug interactions
In FAERS, the use of DPP4is without moderator drugs was significantly associated with reports of rhabdomyolysis (PRR: 2.49, 95%CI: 2.08-2.98). The results showed an increase rhabdomyolysis rate in reports of the concomitant use of DPP4is and moderator drugs, but it was lower than the sum of PRRs for DPP4is and moderator drugs alone, thus did not support a potential drug-drug interaction (Table 1).
| Drug Exposure | Cases (N=28,998) |
Noncases (N= 9,877,644) |
Total | Rate/1000 | PRR (95%CI) |
|---|---|---|---|---|---|
| no DPP4is, no moderator drugs | 7,554 | 7,424,952 | 7,432,506 | 1.02 | Ref |
| DPP4is, no moderator drugs | 119 | 46,994 | 47,113 | 2.53 | 2.49 (2.08-2.98) |
| moderator drugs, no DPP4is | 20,908 | 2,363,509 | 2,384,417 | 8.77 | 8.63 (8.41-8.86) |
| DPP4is and moderator drugs | 417 | 42,189 | 42,606 | 9.79 | 9.63 (8.73-10.63) |
Table 1: The proportional reporting ratios of rhabdomyolysis associated with DPP4is and moderator drugs, separately and in combination, in FAERS
Rhabdomyolysis rates among different population and types of DPP4is
There were a total of 5,878,881 reports that have both age and sex information, therefore we performed similar analysis for different population (ESM Table 2). The rhabdomyolysis rate was 5.18/1000 (95%CI: 3.33-7.04) for DPP4is versus 2.62/1000 (95%CI: 2.52-2.72) for non-DPP4 is, with a corresponding PRR of 1.98 (95%CI: 1.38-2.84) among working age male, while no significant difference was observed in working age female with a PRR of 1.27 (95%CI: 0.61-2.67). However, increase of PRRs was showed in elderly people than working age population, both male and female (Figure 3a). For different types of DPP4is, the PRRs for rhabdomyolysis also varied. Alogliptin, with a PRR of 11.89 (95%CI: 6.77-20.87), was significantly higher than sitagliptin and linagliptin, while saxagliptin and vildagliptin approved to be not associated with rhabdomyolysis after excluding the effects of moderator drugs (Figure 3b).
| Demographics | Drug Exposure | Cases | Noncases | Total | Rate/1000 | PRR (95%CI) |
|---|---|---|---|---|---|---|
| all male | no DPP4is, no moderator drugs | 3,559 | 1,570,534 | 1,574,093 | 2.26 | Ref |
| DPP4is, no moderator drugs | 53 | 11,966 | 12,019 | 4.41 | 1.95 (1.49-2.56) | |
| moderator drugs, no DPP4is | 10,005 | 703,375 | 713,380 | 14.03 | 6.20 (5.97-6.44) | |
| DPP4is and moderator drugs | 221 | 17,555 | 17,776 | 12.43 | 5.50 (4.80-6.29) | |
| working age male | no DPP4is, no moderator drugs | 2,671 | 1,017,467 | 1,020,138 | 2.62 | Ref |
| DPP4is, no moderator drugs | 30 | 5,758 | 5,788 | 5.18 | 1.98 (1.38-2.84) | |
| moderator drugs, no DPP4is | 5,662 | 419,852 | 425,514 | 13.31 | 5.08 (4.86-5.32) | |
| DPP4is and moderator drugs | 68 | 7,262 | 7,330 | 9.28 | 3.54 (2.79-4.50) | |
| elderly male | no DPP4is, no moderator drugs | 888 | 553,067 | 553,955 | 1.60 | Ref |
| DPP4is, no moderator drugs | 23 | 6,208 | 6,231 | 3.69 | 2.30 (1.52-3.48) | |
| moderator drugs, no DPP4is | 4,343 | 283,523 | 287,866 | 15.09 | 9.41 (8.76-10.12) | |
| DPP4is and moderator drugs | 153 | 10,293 | 10,446 | 14.65 | 9.14 (7.71-10.84) | |
| all female | no DPP4is, no moderator drugs | 2,243 | 2,621,442 | 2,623,685 | 0.86 | Ref |
| DPP4is, no moderator drugs | 22 | 12,419 | 12,441 | 1.77 | 2.07 (1.36-3.15) | |
| moderator drugs, no DPP4is | 6,446 | 902,366 | 908,812 | 7.09 | 8.30 (7.91-8.70) | |
| DPP4is and moderator drugs | 148 | 16,527 | 16,675 | 8.88 | 10.38 (8.80-12.25) | |
| working age female | no DPP4is, no moderator drugs | 1,678 | 1,857,081 | 1,858,759 | 0.90 | Ref |
| DPP4is, no moderator drugs | 7 | 6,092 | 6,099 | 1.15 | 1.27 (0.61-2.67) | |
| moderator drugs, no DPP4is | 3,040 | 550,600 | 553,640 | 5.49 | 6.08 (5.73-6.45) | |
| DPP4is and moderator drugs | 59 | 7,128 | 7,187 | 8.21 | 9.09 (7.02-11.77) | |
| elderly female | no DPP4is, no moderator drugs | 565 | 764,361 | 764,926 | 0.74 | Ref |
| DPP4is, no moderator drugs | 15 | 6,327 | 6,342 | 2.37 | 3.20 (1.92-5.34) | |
| moderator drugs, no DPP4is | 3,406 | 351,766 | 355,172 | 9.59 | 12.98 (11.87-14.18) | |
| DPP4is and moderator drugs | 89 | 9,399 | 9,488 | 9.38 | 12.69 (10.16-15.86) |
ESM Table 2: The proportional reporting ratios of rhabdomyolysis associated with DPP4is and moderator drugs, separately and in combination, among different population.
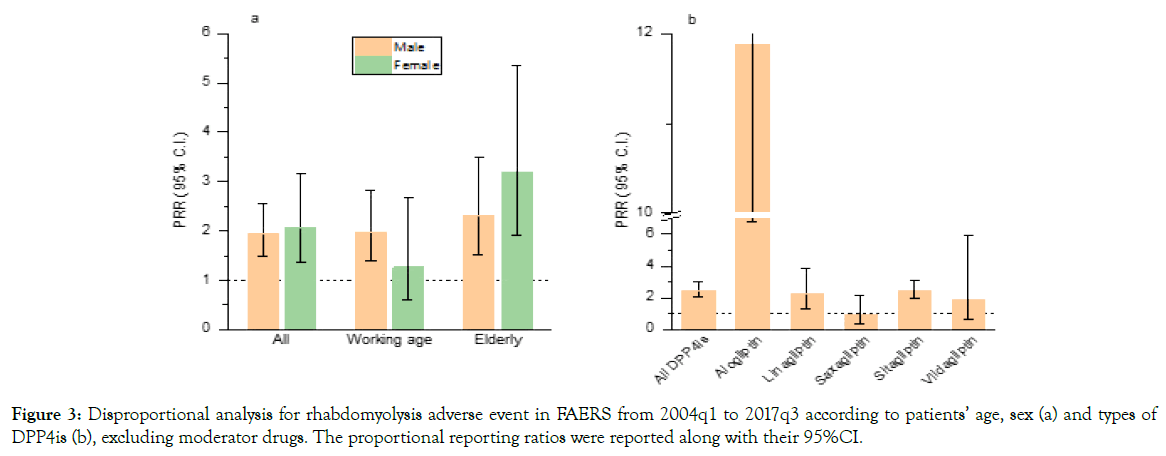
Figure 3. Disproportional analysis for rhabdomyolysis adverse event in FAERS from 2004q1 to 2017q3 according to patients’ age, sex (a) and types of DPP4is (b), excluding moderator drugs. The proportional reporting ratios were reported along with their 95%CI.
Electronic Supplementary Material
Methods
AERSmine QUERY
So far there are five gliptins approved for marketing, alogliptin, linagliptin, saxagliptin, sitagliptin and vildagliptin. According to the AERSmine ontology vocabulary, the query terms listed as follows for each type of DPP4is:
Alogliptin: "alogliptin" OR "metformin and alogliptin" OR "pioglitazone and alogliptin"
Linagliptin: "linagliptin" OR "metformin and linagliptin" OR "linagliptin and empagliflozin"
Saxagliptin: "saxagliptin" OR "metformin and saxagliptin" OR "saxagliptin and dapagliflozin"
Sitagliptin: "sitagliptin" OR "metformin and sitagliptin" OR "pioglitazone and sitagliptin" OR "sitagliptin and simvastatin"
Vildagliptin: "vildagliptin" OR "metformin and vildagliptin"
First, we searched all rhabdomyolysis reports listed DPP4is as suspect or concomitant drug, the search string listed as follows:
Drug: "saxagliptin" OR "metformin and saxagliptin" OR "saxagliptin and dapagliflozin" OR "alogliptin" OR "metformin and alogliptin" OR "pioglitazone and alogliptin" OR "sitagliptin" OR "metformin and sitagliptin" OR "pioglitazone and sitagliptin" OR "sitagliptin and simvastatin" OR "linagliptin" OR "metformin and linagliptin" OR "linagliptin and empagliflozin" OR "vildagliptin" OR "metformin and vildagliptin"
Adverse Events: "rhabdomyolysis"
No restrictions on other variables were used.
Among 536 reports that retrieved using the above query, we listed all drugs that were concomitantly used, and found a total of 60 drugs which were confirmed to be associated with rhabdomyolysis. We defined these 60 drugs as “moderator drugs”.
"valsartan" OR "olmesartan medoxomil" OR "hydrochlorothiazide" OR "pitavastatin" OR "rosuvastatin" OR "irbesartan" OR "losartan" OR "atorvastatin" OR "rabeprazole" OR "pantoprazole" OR "irbesartan and diuretics" OR "ezetimibe" OR "amiodarone" OR "candesartan" OR "tizanidine" OR "simvastatin" OR "pregabalin" OR "aliskiren" OR "olmesartan medoxomil and diuretics" OR "bezafibrate" OR "lovastatin" OR "telmisartan" OR "ciprofloxacin" OR "colchicine" OR "febuxostat" OR "fenofibrate" OR "tolvaptan" OR "theophylline" OR "benzylpenicillin" OR "oxycodone" OR "risperidone" OR "sulfamethoxazole" OR "pravastatin" OR "cefdinir" OR "gemfibrozil" OR "daptomycin" OR "indapamide" OR "temazepam" OR "fluvastatin" OR "ciclosporin" OR "sunitinib" OR "efavirenz" OR "citalopram" OR "sulfasalazine" OR "escitalopram" OR "bupropion" OR "nicotinic acid" OR "pramipexole" OR "lamivudine" OR "aripiprazole" OR "levofloxacin" OR "clarithromycin" OR "terbinafine" OR "cytarabine" OR "venlafaxine" OR "fusidic acid" OR "olanzapine" OR "quetiapine" OR "tenofovir disoproxil" OR "propofol".
Then we performed 4 queries to get the case numbers of rhabdomyolysis associated with DPP4is and moderator drugs, separately and in combination, as follows:
1) No DPP4is, no moderator drugs
Drug:
Include: None.
Exclude: "saxagliptin" OR "metformin and saxagliptin" OR "saxagliptin and dapagliflozin" OR "alogliptin" OR "metformin and alogliptin" OR "pioglitazone and alogliptin" OR "sitagliptin" OR "metformin and sitagliptin" OR "pioglitazone and sitagliptin" OR "sitagliptin and simvastatin" OR "linagliptin" OR "metformin and linagliptin" OR "linagliptin and empagliflozin" OR "vildagliptin" OR "metformin and vildagliptin" OR "valsartan" OR "olmesartan medoxomil" OR "hydrochlorothiazide" OR "pitavastatin" OR "rosuvastatin" OR "irbesartan" OR "losartan" OR "atorvastatin" OR "rabeprazole" OR "pantoprazole" OR "irbesartan and diuretics" OR "ezetimibe" OR "amiodarone" OR "candesartan" OR "tizanidine" OR "simvastatin" OR "pregabalin" OR "aliskiren" OR "olmesartan medoxomil and diuretics" OR "bezafibrate" OR "lovastatin" OR "telmisartan" OR "ciprofloxacin" OR "colchicine" OR "febuxostat" OR "fenofibrate" OR "tolvaptan" OR "theophylline" OR "benzylpenicillin" OR "oxycodone" OR "risperidone" OR "sulfamethoxazole" OR "pravastatin" OR "cefdinir" OR "gemfibrozil" OR "daptomycin" OR "indapamide" OR "temazepam" OR "fluvastatin" OR "ciclosporin" OR "sunitinib" OR "efavirenz" OR "citalopram" OR "sulfasalazine" OR "escitalopram" OR "bupropion" OR "nicotinic acid" OR "pramipexole" OR "lamivudine" OR "aripiprazole" OR "levofloxacin" OR "clarithromycin" OR "terbinafine" OR "cytarabine" OR "venlafaxine" OR "fusidic acid" OR "olanzapine" OR "quetiapine" OR "tenofovir disoproxil" OR "propofol"
2) DPP4is, no moderator drugs
Drug:
Include: "saxagliptin" OR "metformin and saxagliptin" OR "saxagliptin and dapagliflozin" OR "alogliptin" OR "metformin and alogliptin" OR "pioglitazone and alogliptin" OR "sitagliptin" OR "metformin and sitagliptin" OR "pioglitazone and sitagliptin" OR "sitagliptin and simvastatin" OR "linagliptin" OR "metformin and linagliptin" OR "linagliptin and empagliflozin" OR "vildagliptin" OR "metformin and vildagliptin"
Exclude: "valsartan" OR "olmesartan medoxomil" OR "hydrochlorothiazide" OR "pitavastatin" OR "rosuvastatin" OR "irbesartan" OR "losartan" OR "atorvastatin" OR "rabeprazole" OR "pantoprazole" OR "irbesartan and diuretics" OR "ezetimibe" OR "amiodarone" OR "candesartan" OR "tizanidine" OR "simvastatin" OR "pregabalin" OR "aliskiren" OR "olmesartan medoxomil and diuretics" OR "bezafibrate" OR "lovastatin" OR "telmisartan" OR "ciprofloxacin" OR "colchicine" OR "febuxostat" OR "fenofibrate" OR "tolvaptan" OR "theophylline" OR "benzylpenicillin" OR "oxycodone" OR "risperidone" OR "sulfamethoxazole" OR "pravastatin" OR "cefdinir" OR "gemfibrozil" OR "daptomycin" OR "indapamide" OR "temazepam" OR "fluvastatin" OR "ciclosporin" OR "sunitinib" OR "efavirenz" OR "citalopram" OR "sulfasalazine" OR "escitalopram" OR "bupropion" OR "nicotinic acid" OR "pramipexole" OR "lamivudine" OR "aripiprazole" OR "levofloxacin" OR "clarithromycin" OR "terbinafine" OR "cytarabine" OR "venlafaxine" OR "fusidic acid" OR "olanzapine" OR "quetiapine" OR "tenofovir disoproxil" OR "propofol"
3) moderator drugs, no DPP4is
Drug:
Include: "valsartan" OR "olmesartan medoxomil" OR "hydrochlorothiazide" OR "pitavastatin" OR "rosuvastatin" OR "irbesartan" OR "losartan" OR "atorvastatin" OR "rabeprazole" OR "pantoprazole" OR "irbesartan and diuretics" OR "ezetimibe" OR "amiodarone" OR "candesartan" OR "tizanidine" OR "simvastatin" OR "pregabalin" OR "aliskiren" OR "olmesartan medoxomil and diuretics" OR "bezafibrate" OR "lovastatin" OR "telmisartan" OR "ciprofloxacin" OR "colchicine" OR "febuxostat" OR "fenofibrate" OR "tolvaptan" OR "theophylline" OR "benzylpenicillin" OR "oxycodone" OR "risperidone" OR "sulfamethoxazole" OR "pravastatin" OR "cefdinir" OR "gemfibrozil" OR "daptomycin" OR "indapamide" OR "temazepam" OR "fluvastatin" OR "ciclosporin" OR "sunitinib" OR "efavirenz" OR "citalopram" OR "sulfasalazine" OR "escitalopram" OR "bupropion" OR "nicotinic acid" OR "pramipexole" OR "lamivudine" OR "aripiprazole" OR "levofloxacin" OR "clarithromycin" OR "terbinafine" OR "cytarabine" OR "venlafaxine" OR "fusidic acid" OR "olanzapine" OR "quetiapine" OR "tenofovir disoproxil" OR "propofol"
Exclude:"saxagliptin" OR "metformin and saxagliptin" OR "saxagliptin and dapagliflozin" OR "alogliptin" OR "metformin and alogliptin" OR "pioglitazone and alogliptin" OR "sitagliptin" OR "metformin and sitagliptin" OR "pioglitazone and sitagliptin" OR "sitagliptin and simvastatin" OR "linagliptin" OR "metformin and linagliptin" OR "linagliptin and empagliflozin" OR "vildagliptin" OR "metformin and vildagliptin"
4) DPP4is and moderator drugs
Drug:
Include: ("saxagliptin" OR "metformin and saxagliptin" OR "saxagliptin and dapagliflozin" OR "alogliptin" OR "metformin and alogliptin" OR "pioglitazone and alogliptin" OR "sitagliptin" OR "metformin and sitagliptin" OR "pioglitazone and sitagliptin" OR "sitagliptin and simvastatin" OR "linagliptin" OR "metformin and linagliptin" OR "linagliptin and empagliflozin" OR "vildagliptin" OR "metformin and vildagliptin") AND ("valsartan" OR "olmesartan medoxomil" OR "hydrochlorothiazide" OR "pitavastatin" OR "rosuvastatin" OR "irbesartan" OR "losartan" OR "atorvastatin" OR "rabeprazole" OR "pantoprazole" OR "irbesartan and diuretics" OR "ezetimibe" OR "amiodarone" OR "candesartan" OR "tizanidine" OR "simvastatin" OR "pregabalin" OR "aliskiren" OR "olmesartan medoxomil and diuretics" OR "bezafibrate" OR "lovastatin" OR "telmisartan" OR "ciprofloxacin" OR "colchicine" OR "febuxostat" OR "fenofibrate" OR "tolvaptan" OR "theophylline" OR "benzylpenicillin" OR "oxycodone" OR "risperidone" OR "sulfamethoxazole" OR "pravastatin" OR "cefdinir" OR "gemfibrozil" OR "daptomycin" OR "indapamide" OR "temazepam" OR "fluvastatin" OR "ciclosporin" OR "sunitinib" OR "efavirenz" OR "citalopram" OR "sulfasalazine" OR "escitalopram" OR "bupropion" OR "nicotinic acid" OR "pramipexole" OR "lamivudine" OR "aripiprazole" OR "levofloxacin" OR "clarithromycin" OR "terbinafine" OR "cytarabine" OR "venlafaxine" OR "fusidic acid" OR "olanzapine" OR "quetiapine" OR "tenofovir disoproxil" OR "propofol").
Exclude: none.
Discussion
In this study, among the rhabdomyolysis reports for DPP4is, the most commonly represented co-exposed drugs are 3-hydroxy-3- methyl glutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) and Angiotensin II receptor blockers (ARBs), related to abnormal cholesterol and hypertension. With a comparison of rhabdomyolysis rates for DPP4is use with and without moderator drugs, there was no evidence of drug-drug interaction between DPP4is and moderators. This finding is in line with a previous analysis of French Base Nationale de Pharmacovigilance (BNPV) and VigiBase, which suggested that no interaction between DPP4is and statins was found [16]. According to pharmacokinetics characteristics, DPP4is, except saxagliptin, generally do not interfere with cytochrome P450 (CYP) and do not act as inducers or inhibitors of CYP system, suggest that they are not exposed to a high risk of drug–drug interactions [17].
Within the FAERS, DPP4is were disproportionally reported in association with rhabdomyolysis, with a PRR of 2.49 (95%CI 2.08- 2.98), but it was small to be considered robust. According to Huerta- Alardín [18], any drug that impairs the production or use of ATP by skeletal muscle, or increases energy requirements that exceed the rate of ATP production can cause rhabdomyolysis. However, no studies suggested such association at present. In subanalysis, it should be noted that alogliptin has a much higher PRR than other gliptins. As alogliptin was later approved by FDA than sitagliptin (Jan 2013 vs Oct 2006), we recomputed the PRR using data from 2010q2 to 2017q3 (alogliptin was first approved in Japan in April 2010) [19] to exclude potential inflation of PRR (ESM Table 3). The result (PRR: 15.37, 95%CI 8.75-26.99) indicated a solid internal validation for association between alogliptin and rhabdomyolysis. Several literature review [20,21] said alogliptin has no demonstrable advantages over other agents and the side effect profile also does not differ from that of other DPP4is, but according to a comparative study of the binding properties of DPP4is [22], the DPP-4 binding pocket is a large cavity characterized by a certain degree of flexibility, although each of the inhibitors occupy the S1 and S2 sites of the enzymatic cleft, with other areas of the binding site, they showed somewhat different interactions. More dedicated studies are needed to figure out the reason for the difference between alogliptin and other gliptins on rhabdomyolysis.
| Drug Exposure | Cases | Noncases | Total | Rate/1000 | PRR (95%CI) |
|---|---|---|---|---|---|
| non-alogliptin | 4,612 | 5,813,169 | 5,817,781 | 0.79 | Ref |
| alogliptin | 12 | 973 | 985 | 12.18 | 15.37 (8.75-26.99) |
ESM Table 3: The proportional reporting ratios of rhabdomyolysis associated with alogliptin, based on FAERS data from 2010q2 to 2017q3.
Together with the differences of rhabdomyolysis rates among each DDP4i, these data also showed age imbalance, for PRRs in elderly people were higher than that in working age population in male, female and both. This may partly contribute to the characteristics of DPP4is users because the prevalence of T2DM increases markedly with age. On the other hand, analysis based on all rhabdomyolysis AE reports regardless of drugs in FAERS indicated the same trend that the proportion of rhabdomyolysis in elderly people was higher than that in working age people (9,482/1,994,426 vs 13,215/3,884,455). It demonstrated DPP4is associated rhabdomyolysis shared the same characteristics with drug associated rhabdomyolysis (especially statins-induced rhabdomyolysis) on patients’ age [23].
This study has several limitations due to the nature of FAERS data. FDA does not require that a causal relationship between a drug and adverse event be proven, the information in these reports reflects only the reporter's observations and opinions and Submission of a report does not mean that the information included in it has been medically confirmed. Furthermore, FAERS lacks the denominators- the number of patients using the drug. Third, FDA does not receive reports for every adverse event that occurs with a drug because many factors can influence whether an event will be reported, such as the time a product has been marketed and publicity about an event by FDA alerts. Therefore, FAERS data cannot be used to calculate the incidence of an adverse event and make casual inference.
Conclusion
In summary, the analysis indicated that use of DPP4is may be associated with an increased risk of rhabdomyolysis, especially alogliptin. Although causal inference can’t be done and further investigation is warranted, our study is important from a safety prospective and helpful for clinicians to pay attention on the risk of DPP4is-associated rhabdomyolysis.
Acknowledgments
The authors would like to thank Mayur Sarangdhar (Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA) for developing AERSmine.
Sources of Funding
This study was supported by Women's and Children's Health Research Project from Jiangsu Commission of Health (F201867); Project from Department of Science and Technology of Jiangsu Province (BM2018033-2); Young Medical Talent Project from Jiangsu Commission of Health (QNRC2016555); Key Discipline Project on Women's and Children's Health from Jiangsu Commission of Health (FXK201755)
Conflict of Interest
None to report.
REFERENCES
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. Jama. 2007 Jul 11;298(2):194-206.
- Pathak R, Bridgeman MB. Dipeptidyl peptidase-4 (DPP-4) inhibitors in the management of diabetes. Pharmacy and Therapeutics. 2010 Sep;35(9):509.
- White JR. Dipeptidyl peptidase-IV inhibitors: pharmacological profile and clinical use. Clinical Diabetes. 2008 Apr 1;26(2):53-7.
- Richard KR, Shelburne JS, Kirk JK. Tolerability of dipeptidyl peptidase-4 inhibitors: a review. Clinical therapeutics. 2011 Nov 1;33(11):1609-29.
- Karagiannis T, Boura P, Tsapas A. Safety of dipeptidyl peptidase 4 inhibitors: a perspective review. Therapeutic advances in drug safety. 2014 Jun;5(3):138-46.
- Scheen AJ. The safety of gliptins: updated data in 2018. Expert opinion on drug safety. 2018 Apr 3;17(4):387-405.
- US Food and Drug Administration. Potential signals of serious risks/new safety information identified by the FDA Adverse Event Reporting System (FAERS): January–March 2017.
- Kao DP, Kohrt HE, Kugler J. Renal failure and rhabdomyolysis associated with sitagliptin and simvastatin use. Diabetic medicine. 2008 Oct;25(10):1229-30..
- DiGregorio RV, Pasikhova Y. Rhabdomyolysis caused by a potential sitagliptin‐lovastatin interaction. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2009 Mar;29(3):352-6.
- Bhome R, Penn H. Rhabdomyolysis precipitated by a sitagliptin–atorvastatin drug interaction. Diabetic Medicine. 2012 May;29(5):693-4.
- Mor A, Mitnick HJ, Pillinger MH, Wortmann RL. Drug-induced myopathies. Bulletin of the NYU hospital for joint diseases. 2009 Oct 1;67(4):358.
- US Food and Drug Administration. Questions and answers on FDA’s adverse event reporting system (FAERS). Washington: US Department of Health and Human Services. 2018.
- Sarangdhar M, Tabar S, Schmidt C, Kushwaha A, Shah K, Dahlquist JE, Jegga AG, Aronow BJ. Data mining differential clinical outcomes associated with drug regimens using adverse event reporting data. Nature biotechnology. 2016 Jul;34(7):697-700.
- Montastruc JL, Sommet A, Bagheri H, Lapeyre-Mestre M. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. British journal of clinical pharmacology. 2011 Dec;72(6):905.
- Thakrar BT, Grundschober SB, Doessegger L. Detecting signals of drug–drug interactions in a spontaneous reports database. British journal of clinical pharmacology. 2007 Oct;64(4):489-95.
- Labat V, Arnaud M, Miremont-Salamé G, Salvo F, Bégaud B, Pariente A. Risk of myopathy associated with DPP-4 inhibitors in combination with statins: a disproportionality analysis using data from the WHO and French spontaneous reporting databases. Diabetes Care. 2017 Mar 1;40(3):e27-9.
- Scheen AJ. Pharmacokinetics of dipeptidylpeptidase‐4 inhibitors. Diabetes, Obesity and Metabolism. 2010 Aug;12(8):648-58.
- Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: Rhabdomyolysis–an overview for clinicians. Critical care. 2004 Apr 1;9(2):158.
- Days CD. Preliminary Considerations. 2011
- Andukuri R, Drincic A, Rendell M. Alogliptin: a new addition to the class of DPP-4 inhibitors. Diabetes, metabolic syndrome and obesity: targets and therapy. 2009;2:117.
- Jarvis CI, Cabrera A, Charron D. Alogliptin: a new dipeptidyl peptidase-4 inhibitor for type 2 diabetes mellitus. Annals of Pharmacotherapy. 2013 Nov;47(11):1532-9.
- Berger JP, SinhaRoy R, Pocai A, Kelly TM, Scapin G, Gao YD, Pryor KA, Wu JK, Eiermann GJ, Xu SS, Zhang X. A comparative study of the binding properties, dipeptidyl peptidase‐4 (DPP‐4) inhibitory activity and glucose‐lowering efficacy of the DPP‐4 inhibitors alogliptin, linagliptin, saxagliptin, sitagliptin and vildagliptin in mice. Endocrinology, diabetes & metabolism. 2018 Jan;1(1):e00002.
- Schech S, Graham D, Staffa J, Andrade SE, Grenade LL, Burgess M, Blough D, Stergachis A, Chan KA, Platt R, Shatin D. Risk factors for statin‐associated rhabdomyolysis. Pharmacoepidemiology and drug safety. 2007 Mar;16(3):352-8.
Citation: Wenhui S, Lei B, Zhiming S. (2020) A Disproportionality Analysis of the Risk of Rhabdomyolysis Associated with DPP-4 Inhibitors in the Food and Drug Administration Adverse Event Reporting System J. Pharamacovigil. 9:295. doi-10.35248/2329-6887.20.9.295.
Copyright: © 2020 Wenhui S et al This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


