PMC/PubMed Indexed Articles
Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
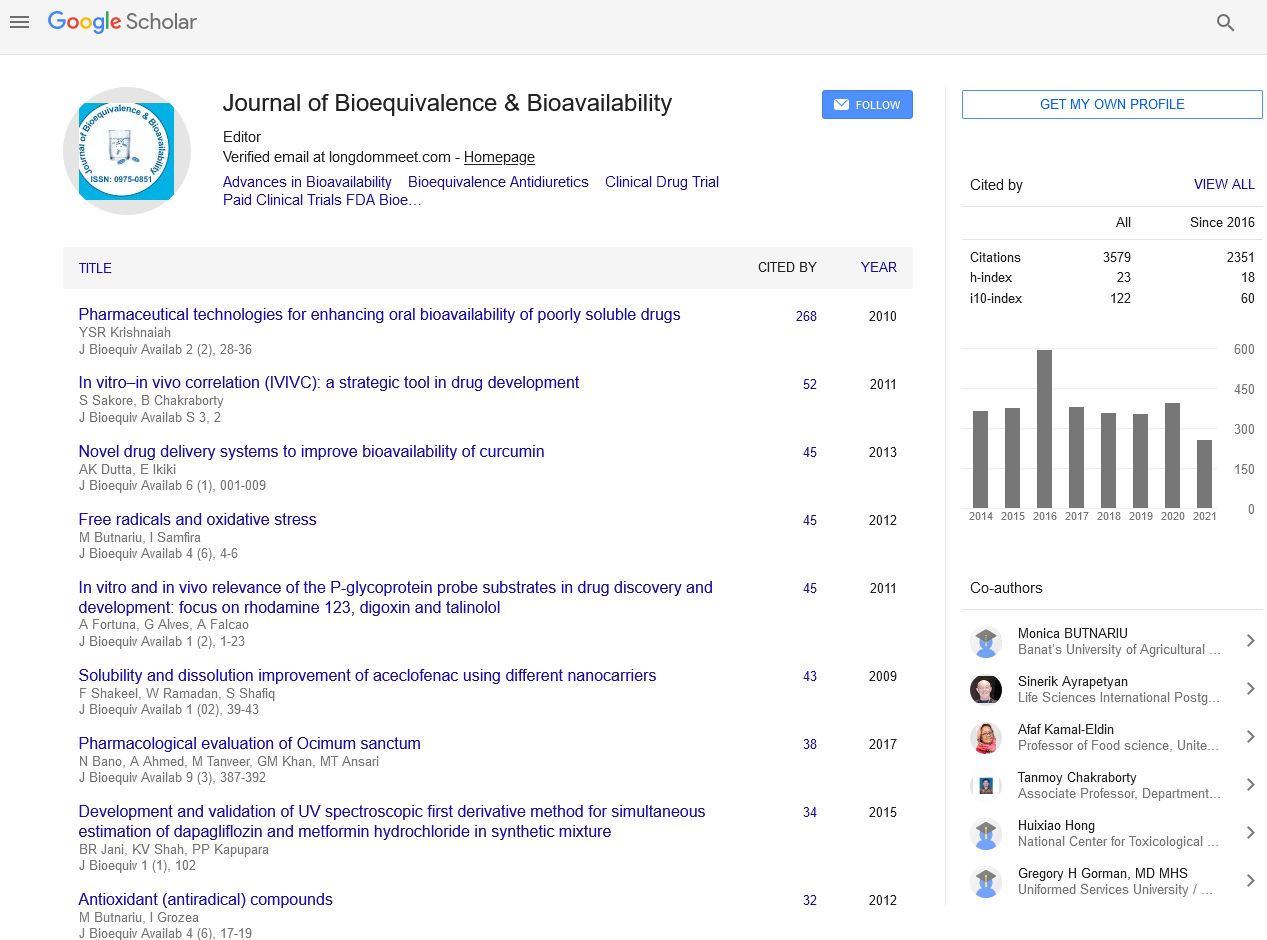
Articles published in Journal of Bioequivalence & Bioavailability have been cited by esteemed scholars and scientists all around the world. Journal of Bioequivalence & Bioavailability has got h-index 26, which means every article in Journal of Bioequivalence & Bioavailability has got 26 average citations.
Following are the list of articles that have cited the articles published in Journal of Bioequivalence & Bioavailability.
| 2022 | 2021 | 2020 | 2019 | 2018 | |
|---|---|---|---|---|---|
Year wise published articles |
60 | 61 | 19 | 6 | 27 |
Year wise citations received |
394 | 412 | 405 | 380 | 363 |
| Journal total citations count | 4363 |
| Journal Impact Factor | 6.89 |
| Journal 5 years Impact Factor | 11.60 |
| Journal CiteScore | 14.43 |
| Journal h-index | 26 |
Important citations
Impact of Pluronics of different structure on pharmacologically relevant properties of sulfasalazine and methotrexate
Protective effects of gallic acid against methotrexate-induced toxicity in rats
Hepatoprotective potential of Rosmarinus officinalis essential oil against hexavalent chromium-induced hematotoxicity, biochemical, histological, and immunohistochemical changes in male rats
Hepatoprotective potential of Rosmarinus officinalis essential oil against hexavalent chromium-induced hematotoxicity, biochemical, histological, and immunohistochemical changes in male rats
Protective effects of gallic acid against methotrexate-induced toxicity in rats
Impact of Pluronics of different structure on pharmacologically relevant properties of sulfasalazine and methotrexate
Spirulina ameliorates methotrexate hepatotoxicity via antioxidant, immune stimulation, and proinflammatory cytokines and apoptotic proteins modulation
Spirulina ameliorates methotrexate hepatotoxicity via antioxidant, immune stimulation, and proinflammatory cytokines and apoptotic proteins modulation
Methotrexate-loaded metal-organic frameworks on the basis of ?-cyclodextrin: Design, characterization, in vitro and in vivo investigation
Methotrexate-loaded metal-organic frameworks on the basis of ?-cyclodextrin: Design, characterization, in vitro and in vivo investigation
Spirulina ameliorates methotrexate hepatotoxicity via antioxidant, immune stimulation, and proinflammatory cytokines and apoptotic proteins modulation
Comparative Analysis Between Training Methods for a Fuzzy Inference System Potentially Applicable to the Assessment of the Health Status of the Spine in Military Personnel
Preparation and characterisation of Kolliphor® P 188 and P 237 solid dispersion oral tablets containing the poorly water soluble drug disulfiram
Approaches to increase mechanistic understanding and aid in the selection of precipitation inhibitors for supersaturating formulations – a PEARRL review
Synthon polymorphs of 1?:?1 co-crystal of 5-fluorouracil and 4-hydroxybenzoic acid: their relative stability and solvent polarity dependence of grinding outcomes†
Synthon polymorphs of 1?:?1 co-crystal of 5-fluorouracil and 4-hydroxybenzoic acid: their relative stability and solvent polarity dependence of grinding outcomes†
The Effect of Non-Thermal Microwave-Treated Physiological Solution on Isolated Heart Muscle of Snail
Using USP I and USP IV for Discriminating Dissolution Rates of Nano- and Microparticle-Loaded Pharmaceutical Strip-Films
Preparation, in-vitro and in-vivo characterisation of CoQ10 microparticles: electrospraying-enhanced bioavailability
Preparation, in-vitro and in-vivo characterisation of CoQ10 microparticles: electrospraying-enhanced bioavailability