Modification of Seeds to Roots: The Uniqueness of Germination and Growth of Putat (Barringtonia acutangula)
Abstract
Putat (Barringtonia acutangula L. Gaertn) is well-adapted to high and low tide conditions. Putat has a high antioxidant content, so it can overcome various health problems. However, research on the adaptation of putat to water conditions and the germination and growth of putat seedlings still needs to be completed. This study aims to analyze the seed germination process and development of putat seedlings morphologically. The results showed that putat undergoes an unusual and unique germination process. Its shoot and root grow at different seed poles; the node comes out at the base, while the taproot comes out at the tip. As the seeds germinate, the fruit skin remains attached to the sources. After expanding into seedlings, the seeds are modified into roots, thus connecting the taproot that appears first at the end of the basis with the stem that grows at the base.
Keywords
Barringtonia acutangula, putat, seed germination, seedling growth
Introduction
Putat is the local name for plants in the genera Planchonia and Barringtonia. This plant belongs to the Lecythidaceae family, and one of the species is B. acutangula. Putat spread across Southeast Asia with tropical climates, such as Laos, Thailand, Myanmar, Malaysia, the Philippines, Vietnam, and Indonesia. The plant also grows in Australia, Tibet, India, and Nepal (Quattrochi, 2016).
Putat trees grow well in open environments or environments with high light intensity. However, this plant can also live in the shade. (Syukur 2016) reported that putat is the dominant vegetation in the Jemelak River, Sintang District, West Kalimantan, Indonesia. The biotic and abiotic factors of the Jemelak River are very suitable for the putat. In addition, putat can grow in various types of soil and adapt well to tidal conditions. A different species of putat, namely B. asiatica has leaf adaptations with specialized mesophyll tissue. This adaptation causes the species to be able to live in an environment without shade. However, the putat species cannot adapt to dry tropical climates (Rendyastuti and Hapsari, 2017).
The knowledge about the germination process of putat that commonly grows in a riparian area is limited. When its fruits ripen and fall into the water, they will sink for a certain period, so the putat germination will occur in a submerged condition. However, many factors influence the germination rate of putat, including seed weight. (Nath et al. 2016a) observed that seeds belonging to the heavy class (23 g) have a fast growth rate compared to those from the medium (2g-3 g) and the light (1g-2 g) classes. Heavyweight seeds germinate faster and have higher shoots than the other two seed classes. In addition, the water content also affects the number of seedling growth from the putat seeds. Seeds with a heavy-weight class have more critical values for conserving future putat populations.
The germination and growth of putat seedlings are also affected by the concentration of heavy metals which contaminate the water and soil. According to (Seneviratne et al. 2017), soil contaminated with heavy metals harms seed germination and seedling growth. The environment contaminated with heavy metals inhibits the activities of hydrolysis enzymes that play a role in the germination process. One example is cadmium (Cd) which inhibits carbohydrate hydrolysis, thereby reducing the growth activity of seedlings.
Heavy metals such as cadmium, lead, and cobalt also affect the development of pollen and pollen tubes of putat flowers. High concentrations of heavy metals cause anomalies in the pollen tube and pollen putat morphology. Imperfect development and growth of pollen will affect the pollination process. They will also affect productivity, reproduction, and putat plant populations (Ghanta & Mandal, 2016). Other factors that affect putat reproduction are rainfall, temperature, and drought. Climate change will significantly affect putat reproduction and its natural distribution (Nath et al.2016b; Deb et al. 2016).
Local people in the research site around Tangkas Lake, commonly consume the leaves of putat as a raw vegetable to increase palatability. In India and Bangladesh, putat has long been known as traditional medicine (Kaur et al., 2013; Faruk et al., 2016). Leaves, roots, tree bark, and seeds of the putat have a high antioxidant content and can address various health problems. From an ecological perspective, putat acts as a shelter for fish from predators during high tide.
The leaves and bark of the putat (B. acutangula) contain phenols and flavonoids. Based on its bioactive compounds, this plant is an antioxidant and antimicrobial (Mohan & Anand, 2019). Meanwhile, another species of putat Planchonia valida also contains alkaloids, flavonoids, tannins, saponins, and steroids (Supriningrum et al., 2019). The content of saponins in the bark and seeds of B. acutangula is the potential to treat diabetes mellitus, obesity, and metabolic syndrome (Patil & Khatib, 2020).
The antioxidant content in putat leaves has the potential to be developed as a tea product to support the local community's economy. The Tangkas Lake is one of the putat habitats in Jambi Province, Indonesia. Villagers began to learn how to process putat leaves into tea. The community is committed to producing tea from putat leaves through counseling and training to improve the local economy (Tarigan et al., 2022).
The increasing use of putat and the impact of climate change in the future will threaten the existence of putat. The impact could be minimized by a comprehensive conservation effort effectively implemented on the ground. However, the putat adaptation to a periodically inundated (riparian) environment and its germination process is unknown. The research aims to analyze seed germination and growth of putat seedlings (B. acutangula) in their natural habitat.
Methods
We conducted the research at Tangkas Lake in Tanjung Lanjut Village, Sekernan District, Muaro Jambi Regency, Jambi Province, Indonesia, from February to March 2023. This study used a qualitative approach to describe the conditions of the samples found at the research site. The researcher collected samples by exploring the Tangkas Lake area when the water receded. Tangkas Lake is a riparian lake that experiences ebb and flow following the water level of a nearby Kaos River. The samples collected were putat shoots and saplings with different morphology, old putat fruits taken directly from the tree. The research selected samples by considering their morphological appearance for both the sprout’s and seedling’s states. The difference in the height of sprouts and seedlings indicated an age difference. A taller seedling in height indicated to have an older age.
Furthermore, we observed the morphology of sprouts and seedlings. They included observations fruit bunches from about 50 individual trees. We observed the growth of 25 individual sprouts and seedlings in more detail.
Research data were analyzed descriptively by describing the morphology of putat sprouts and seedlings. Morphological characters observed included fruit shape and texture, root system, shoot growth, and other morphological characters at each stage of putat seed development. We analyzed the results of morphological observations of seedlings with different heights to obtain a general description of the growth rate of seedlings based on their height. The results describe the morphological phenomena of germination and growth of putat seedlings.
Results and Discussion
Observations on many samples collected from the field, one putat fruit bunch generally consists of eight mature fruits. Further observations of fruit, sprouts, and seedlings at various growth stages showed a unique phenomenon in either seeds, sprouts, or putat seedlings. Each part showed remarkable morphological differences compared to typical plant morphology (Tab. 1).
Table 1. Differences in the morphology of seeds, sprouts, and putat seedlings compared to the typical plant morphology
| Unique Putat Morphologies | Typical Plant Morphologies |
|---|---|
| Seeds | |
| Transverse and longitudinal sections show no embryo. | Shows the presence of an embryo. |
| The texture of the seeds is smooth, in the form of a homogeneous mass, dense and tough, and does not show the presence of cotyledons. | Shows the presence of cotyledons. |
| Sprouts | |
| The seeds are still in the fruit skin because they do not come off when they germinate. During germination, the fruit skin will dry out, become thinner, and change color to blackish brown. | When the seeds start germinating, they detach from the fruit; thus, they are clearly visible. |
| Shoots and roots grow at opposite poles of the seed. Shoots appear at the seed's base, and the taproot comes out at the tip of the seed . As a result, the node does not directly connect to the root; instead, connected by a modified seed to the root. | Shoots and roots grow at the same pole of the seed, so that the shoot is directly connected to the root. |
| Seedlings may be mono-embryonic (producing one bud) or polyembryonic (having more than one shoot). | A plant species generally has only one germination trait, namely, mono-embryonic or polyembryonic. |
| Polyembryonic sprouts produce more than one shoot but have only one taproot. | Each shoot has one taproot. |
| Seedlings | |
| Seeds are still visible, but the fruit skin that covers them is thinner and shows cracks. Then, the skin sloughs off, and the seeds are modified to become a part of the taproot. | The seeds are no longer there because the seed coat is released at the time of germination; the cotyledons shrink, dry out and then fall off while the embryo develops into seedlings. |
| Modified seeds become roots, connecting the stem with the taproot that has grown at the start of germination. | Roots and shoots are interconnected and grow into seedlings, with no part of the seed separating the node from the root. |
| Taproot continues to grow and produce root branches at the end of the seed. Additional roots (adventitious roots) also rise in the middle and base of the seed (base area of the stem); thus, adventitious roots grow spread over the entire surface of the seed . | Roots continue growing and form root branches to form a taproot system in dicot plants. In monocot plants, the roots that emerge during germination dry out and die. Furthermore, adventitious roots grow, which then form a fibrous root system. |
Putat fruit collected directly from the tree has an oval shape with four wings. Fruit skin is textured hard and relatively thick. When it is ripe, the skin color of the fruit is brown. The fruit is not stalked and grows dangling on tree branches near the water's surface (Fig. 1a). At a certain period, the putat fruit will separate from the primary fruit stalk and fall into the lake's waters. How long it took the fruit to reach the bottom of the lake has yet to be determined.
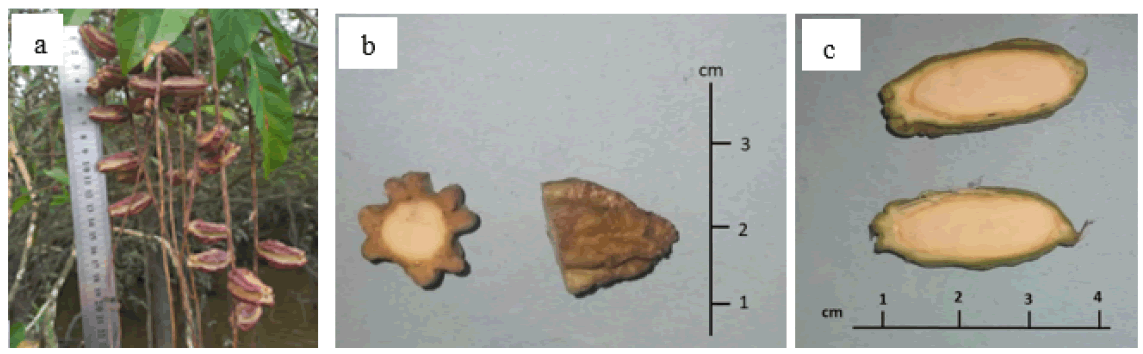
Figure 1: Putat Fruit: a. The putat fruit dangles on its tree; b. Cross section; c. Longitudinal cut
The cross-section of the fruit is round, with four distinct wings. The seeds inside the fruit are white (Fig. 1b). The longitudinal section clearly shows the brown line between the rind and the inside. The fruit skin, seed coat, and putat seeds are attached. In addition, there was no visible embryo in the seeds. The cut surface of the fruit, transversely and longitudinally, shows a homogeneous structure and texture. Seeds are not split; showed a monocotyledon seed-like morphology (Fig. 1c).
Seeds in dicot plants generally have three parts, and one example is the kuku wood tree (Pericopsis mooniana THW). Furthermore, (Suhartati et al. 2015) reported that the structure of kayu kuku seed consists of the seed coat, the umbilical cord (Funiculus) and the seed hilum (Hilus), and the seed core. The seed coat consists of two parts, namely the outer layer (Testa) and the inner layer (Tegmen). Meanwhile, the seed core consists of the embryo, seed roots (Radiculus), seed leaves (Cotyledon), and stem seed (Caulicus).
Generally, the morphology of the seeds is visible because they have separated from the fruit skin. Furthermore, the seeds will enter the germination process when environmental conditions are favorable. However, germinating putat seeds takes place slightly differently from other types of plants in general. Observation of putat seed germination that falls into the waters is challenging. Samples found at the study site showed putat growth at the germination stage. Putat seeds are immersed, and only shoots can be seen on the surface (Fig. 2).
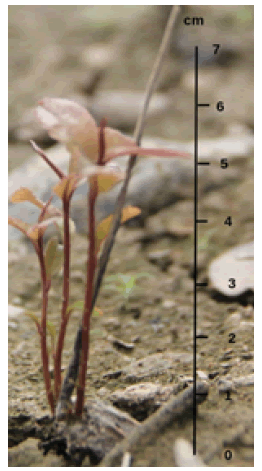
Figure 2: Samples of putat seeds found at low tide show the stages of shoot growth
The observed putat sprouts and seedlings showed they were in different growth phases. This finding implies that putat fruits sinking and reaching the lake's bottom occurs at different times. Moreover, the putat shoots and seedlings collected were from different ripening periods or fruiting seasons. (Aluri et al. 2019) say putat fruit has a single seed. When ripe, the fruit falls into the water and sinks to the ground. The seeds will start to germinate and produce seedlings within 2 weeks-3 weeks.
Germination is the process of growth and development of embryos in plant seeds that have passed a dormant period (Fig. 3). The germination process begins with imbibition, water absorption by plant seeds. Seed morphology is an essential factor in the imbibition process. The Artabotrys hexapetalus is an example of a plant that takes a long time to germinate. The seeds have hard skin with three layers (Exotesta, mesotesta and endotesta) and have endosperm with a hard texture (Handayani, 2017).
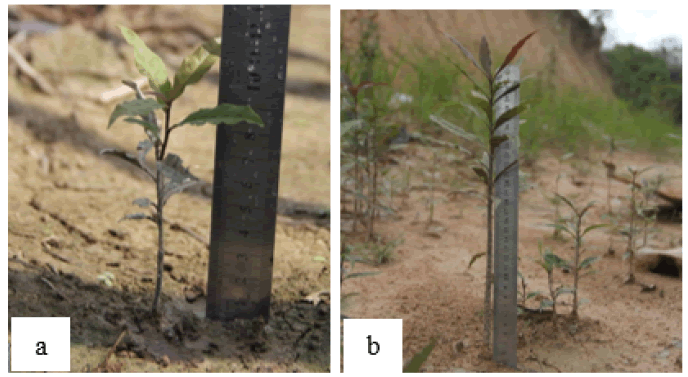
Figure 3: Putat sprouts and seedlings: a. Young putat seedlings (lower); b. Older Putat seedlings (higher)
In addition to seed morphology, environmental factors such as light, water availability, temperature, and soil conditions influence germination. Each type of plant has different needs for these factors. Plants living in muddy, swampy, and shallow water need light for germination. Meanwhile, changing temperatures can help plant germination, mainly in muddy soils (Rosbakh et al., 2020).
After the seeds pass through the imbibition process, certain parts of the seeds will start to crack. Roots began to appear, and shoots began to come out on the soil surface. Several plant species have different germination types and processes. Candlenut seeds, with a hard skin texture, have a hypogeal germination type. Their cotyledons do not grow large but remain in the soil. Their hypocotyl, which appears first, develops into roots and shoots (Susilowati et al., 2020). The seeds of the Euchresta horsfieldii also belong to the hypogeal germination type. Germination begins with the emergence of radicles, followed by the growth of their epicotyl. Then, the radicle develops into roots, while the epicotyl grows into leaves (Kuswantoro & Oktavia, 2019). Based on the observation of the condition of the putat germination, the putat seeds also belong to the hypogeal germination type. Its seeds remain in the soil during the germination process.
In general, shoots and roots will grow on the same seed pole. However, the putat seed samples showed a different germination morphology than common seed plants. The shoot and root of the putat grow at opposite poles. Shoots grow at the base of the seed, while roots grow at the tip of the seed. The root that emerges from the tip of the seed is a taproot (Fig. 4a). Unlike common plant seeds, putat seeds can produce one to three shoots; thus, putat can have the properties of mono-embryonic seeds (Fig. 4b) or polyembryonic seeds (Fig. 4c). Putat shoots that grow on a single seed use a single taproot.

Figure 4: Putat seed germination: a. Putat germinates with roots and shoots growing on opposite poles of the seed; b. Putat germination as mono-embryonic seedlings; c. Germination of putat as polyembryonic seeds.
The polyembryonic nature of the putat can originate from the differentiation of somatic cells or seed tissue. Cut crosswise and longitudinally of the putat seeds' structure showed a homogeneous seed texture and did not indicate the presence of any embryos. The shoots and roots that appear when the seed germinates do not originate from the embryo but from the somatic cells or tissues of the seed. We expected that putat reproduction occurs through an asexual process (Apomixis). However, further research is needed to clarify, especially regarding to the biology of putat flowers. The phenomenon of polyembryonic is also found in other types of plants, including plants adapted to terrestrial environments, such as duku (Lansium parasiticum) and squeezed oranges (Citrus sinensis L.).
Research by (Murni et al. 2019) on the duku showed that its seeds are polyembryonic because they reproduce asexually through seeds. Genetic variation from the offspring and the parent tree shows homogeneous genetics. Embryos found in seeds are known to be somatic. In contrast to putat, duku seeds show a transparent embryo. One seed contains 1 shoot-6 shoots, each with a taproot. Another case of polyembryony occurs in squeezed oranges. Seeds of the orange are polyembryonic due to the presence of nucellar embryos in seeds that develop from somatic cells of the female parent. The sprouts and seedlings that grow have the same appearance as the female parent, and their growth is relatively faster and more uniform (Wahyudhi, 2020).
While still in the germination stage, roots function as a means of absorbing water and nutrients. Shoots will continue to grow if the roots can develop and function properly. The first root that grows when the seed germinates is the primary root. This root will develop into a taproot as the primary root system in dicot plants. The bigger the plant, the more roots develop and produce many smaller branches. Thus, the parts of the roots in dicotyledonous plants consist of a root base, taproot, root branches, and root caps (Susetyarini et al., 2020).
In certain plants, roots might modify into various forms according to their surrounding environmental conditions. Mangrove plants are among the plants that have various forms of roots, including knee roots, buttress roots, and respiratory roots. Bruguiera gymnorrhiza is a species of mangrove plant that has knee roots. Roots of the species continue to grow sideways with root lengths that can reach 12 meters. Meanwhile, the Rhizophora mucronate, another mangrove species, has supporting roots that can grow up to 2.5 meters in height (Idrus et al., 2014).
Even though similar to mangrove plants adapted to aquatic environments, the form of adaptation of putat roots is uncertain. However, the morphology of the putat root does not differ much from other dicot plants, which have a taproot. Yet, the development of the growth of putat roots is different from plants in general. Putat roots develop from seeds that cannot be separated from the fruit when germinating. At a later stage, the fruit skin will thin and show cracks when the putat is in the growth phase from sprouts to seedlings.
During the seedling growth phase, the seed coat gets thinner and peels off, and the seed continues to modify to become part of the taproot (Fig. 5a); the primary root that grows at the end of the seed, continues to elongate, enlarge, and produce lateral branches. Modified seeds become roots, connecting the stem and primary roots. When the putat seedlings get more extensive and taller, the fine roots continue to fill the root starting from the root's base, middle, and tip (Fig. 5b). (Fig. 6) overviews the morphological phenomena of germination and growth of putat seedlings at different age levels.

Figure 5: Putat seedlings: a. Seed coat that is thinned and cracked; b. Modification of the seed becomes a part of the taproot.

Figure 6: Stages of putat growth from sprouts to seedlings
Germination and growth of putat seedlings took place in four stages. The first stage begins with the imbibition process, the seeds absorb water, and the dormant period ends. In the second stage, seeds begin to show primary roots and shoots. The third stage is the growth of primary roots, which are getting bigger and forming a taproot system. At this stage, the shoots also appear to get bigger and develop into a stem overgrown with several leaves. In the last stage, the seeds modify to become a part of the taproot.
Conclusion
Putat is a tree that has an excellent adaptation to the riparian environment. The skin of the fruit is hard-textured and relatively thick. When ripe, the fruit will be brown. Seeds germinate uniquely by showing roots' growth and shooting at opposite poles. At later stages of growth, the fruit skin remains attached to the seeds and grows into seedlings. Seeds undergo modification into roots, connecting the taproot that has grown at the ends. The number of shoots that grow on one seed can be one or more; however, the growing taproot remains one. Moreover, transverse and longitudinal sections of the seeds showed no embryos.
Acknowledgement
The authors thank the Directorate of Research, Technology, and Community Development, Indonesian Ministry of Education, for providing funds through the Higher Education Excellence Basic Research scheme with Number SP DIPA-023.17.1.690523/2022 dated November 17th, 2021.
References
Aluri, J.S.R., Palathoti, S.R., Banisetti, D.K., Samareddy, S.K. 2019. Pollination Ecology Characteristics of Barringtonia racemora L. Spreng. (Lecythidaceae). Transylv Rev Syst Ecol Res. 21:27-34. [Google Scholar] [Cross Ref]
Deb, J. C., Rahman, H. T., & Roy, A. 2016. Freshwater swamp forest trees of Bangladesh face extinction risk from climate change. Wetlands. 36: 323-334. [Google Scholar] [Cross Ref]
Faruk, M.O., Sardar, R., Haque, S. T., & Haque, M.E. 2016. Antimicrobial, cytotoxic and antioxidant activities of Barringtonia acutangula (L). Bioresearch Commun.-(BRC),2: 205-209. [Google Scholar] [Cross Ref]
Ghanta, R. dan Mandal, S. 2016. Effect of Some Heavy Metals on Pollen Viability of Barringtonia acutangulaGaertn. J. Palynol. 52: 37-47. [Google Scholar] [Cross Ref]
Handayani, T. 2017. Seed Germination and Seedling Morphology of Artabotrys hexapetalus. Nusant. Biosci. 9: 23-30. [Google Scholar] [Cross Ref]
Idrus, A.A., Mertha, I.G., Hadiproyitno, G., Ilhamdi, M.L. 2014. Kekhasan Morfologi Spesies Mangrove di Gili Sulat (The uniqueness of mangrove species morphology in the Gili Sulat). J. Biol. Trop. 14: 120-128. [Google Scholar] [Cross Ref]
Kaur, M., Singh, G., Mohan, C. 2013. Barringtonia acutangula: A traditional medicinal plant. Int J Pharm Sci Rev Res. 23: 168-171. [Google Scholar] [Cross Ref]
Kuswantoro, F., Oktavia, G.A.E. 2019. Studi Tipe Perkecambahan dan Pertumbuhan Anakan Pinanga arinasae Witono dan Euchresta horsfieldii (Lesch.) Benn. Untuk Mendukung Upaya Konservasinya (Germination and Growth Type Study of Pinanga arinasae Witono and Euchresta horsfieldii (Lesch.) Benn. to Support Its Conservation Efforts). Bul Kebun Raya. 12: 21-32. [Google Scholar] [Cross Ref]
Mohan, S.C., Anand, T. 2019. Comparative Study of Identification of Bioactive Compounds from Barringtonia acutangula Leaves and Bark Extract and its Biological Activity. Appl Sci., 19: 528-536. [Google Scholar] [Cross Ref]
Murni, P., Syamsuardi., Nurainas., Mansyah, E., Huda, M. 2019. The Absolute Genetic Invariability of Polyembryony Seedling of Duku ‘Kumpeh’ (Lansium parasiticum (Osbeck) K.C. Sahni & Bennet.) (Lansium parasiticum (Osbeck) K.C. Sahni & Bennet.) A Superior Type from Jambi, Indonesia. SABRAO J Breed Genet. 51: 151-160.[Google Scholar] [Cross Ref]
Nath, S., Nath, A.J., Das, A.K. 2016. Seed Germination in Barringtonia acutangula: A Floodplain Tree From North East India. Int J Ecol Environ Sci. 42: 47-53.[Google Scholar] [Cross Ref]
Nath, S., Nath, A.J., Das, A.K. 2016. Vegetative and Reproductive Phenology of a Floodplain Tree Species Barringtonia acutangula from North East India. J Enviromental Biol.. 37: 215-220. [Google Scholar] [Cross Ref]
Patil, V.S., Khatib, N.A. 2020. Triterpene Saponins from Barringtonia acutangula (L.) Gaertn as a Potent Inhibitor of 11β-HSD1 for Type 2 Diabetes Mellitus, Obesity and Metabolic Syndrome. Clin Phytoscience. 6: 1-5. [Google Scholar] [Cross Ref]
Quattrocchi, U. 2016. CRC World Dictionary of Medical and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms and Etymology(5 Volume Set). U K: CRC Press. [Google Scholar] [Cross Ref]
Rendyastuti, R., Hapsari, L. 2017. Adaptasi Ekofisiologi Terhadap Iklim Tropis Kering: Studi Anatomi Daun Sepuluh Jenis Tumbuhan Berkayu (Ecophysiological Adaptation to Dry Tropical Climate: Study of Leaf Anatomy of Ten Types of Woody Plants). J Biol Indones. 13: 1-14. [Google Scholar] [Cross Ref]
Rosbakh, S., Phartyal, S.S., Poschlod, P. 2020. Seed Germination Traits Shape Community Assembly Along a Hydroperiod Gradient. Ann Bot. 125: 67-78. [Google Scholar] [Cross Ref]
Seneviratne, M., Rajakaruna, N., Rizwan, M., Madawala, H.M.S.P., Ok, Y.S., Vithanage, M. 2017. Heavy Metal-Induced Oxidative Stress on Seed Germination and Seedling Development: A Critical Review. Environ Geochem Health. 41: 1813-1831. [Google Scholar] [Cross Ref]
Suhartati, N., Alfaizin, D. 2015. Mengenal Morfologi, Tipe Buah dan Biji Pada Pohon Kayu Kuku (Pericopsis mooniana THW) (Understanding Morphology, Types of Fruits, and Seeds on Kuku Wood Trees (Pericopsis mooniana THW). Info Teknis Eboni. 12: 87-96. [Google Scholar] [Cross Ref]
Supriningrum, R., Fatimah, N., Purwanti, Y.E. 2019. Karakteristik Spesifikasi dan Non Spesifikasi Ekstrak Etanol Daun Putat (Planchonia valida) (Specification and Non-Specification Charactersitic of Putat Leaf (Planchonia valida) Ethanol Extract). Al Ulum Sains dan Teknologi. 5: 6-12. [Google Scholar] [Cross Ref]
Susetyarini, E., Wahyono, P., Latifa, R., Nurrohman, E. 2020. The Identification of Morphological and Anatomical Structure of Pluchea indica. J Physic: Conf Ser. 1539: 1-13. [Google Scholar] [Cross Ref]
Susilowati, A., Dalimunthe, A., Rachmat, H.H., Elfiati, D., Srimambela, P.Y., Ginting I.M., Larangkeng, S.H. 2020. Morphology and Germination of the Candlenut Seed (Aleurites moluccana) From Samosir Island, North Sumatra. Conf Ser: Earth Environ Sci. 454: 1-5. [Google Scholar] [Cross Ref]
Syukur, M. 2016. Habitat Putat (Barringtonia acutangula) Pada Kawasan Berhutan Sungai Jemelak Kabupaten Sintang (Putat Habitat (Barringtonia acutangula) in the Forested Area of the Jemelak River, Sintang District). PIPER. 23(12): 74-82.
Tarigan, I.L., Hariyadi, B., Pebridayanti, P., Latief, M. 2022. Pemanfaatan Tanaman Putat Sebagai Teh Fungsional dalam Mendukung Desa Ekowisata Danau Tangkas Desa Tanjung Lanjut (Utilization of Putat Plants as Functional Tea in Supporting the Danau Tangkas Ecotourism Village, Tanjung Lanjut Village). Jurnal Pengabdian Pada Masyarakat. 7(4): 842-850.
Author Info
Faculty of education and teacher training, UniversityJambi, IndonesiaFaculty of science and Technology, University Jambi, Indonesia
Received: 25-Apr-2023, Manuscript No. mp-23-97018; Editor assigned: 27-Apr-2023, Pre QC No. mp-23-97018(PQ); Reviewed: 01-May-2023, QC No. mp-23-97018(Q); Revised: 10-May-2023, Manuscript No. mp-23-97018(R); Accepted Date: 19-May-2023 Published: 30-May-2023